French study shows COVID vaccine efficacy 94% against severe outcomes
In late December 2019, France began administering vaccinations against coronavirus disease 2019 (COVID-19) to its population. At the end of 2021, 77% of the entire population were fully vaccinated, and 91% of those 18 years or older. During these times, France experienced three epidemic waves caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants Alpha, Delta, and Omicron.
Vaccination administration was stratified among the different population groups due to limited availability, begging with those at most risk of severe COVID-19-related complications and those who work in healthcare. Moderna, Pfizer/BioNTech, AstraZeneca, and Janssen were the four Covid-19 vaccine brands utilized. With the exception of the Janssen vaccine, which only required one dosage, a vaccination program was initially considered complete following two doses.
A complete vaccination cycle had become a prerequisite for obtaining a French health permit, which became effective in June and was first necessary to enter events and places with large crowds. In August, the health pass was expanded to include admission to all museums, bars, restaurants, railways, and other public venues.
Evidence began to point to a significant drop in vaccine effectiveness towards symptomatic disease over time, but only to a smaller scale against severe infections. For example, vaccine effectiveness against symptomatic Delta variant infections declined to 47% and 70%, respectively, 20 weeks after immunization for AstraZeneca and Pfizer/BioNTech vaccines, with a higher reduction in individuals aged 65 and older, but a lesser reduction against hospitalizations. Nevertheless, studies show that the booster dose recovers protection against symptomatic infections and severe cases to levels similar to those seen before immunity waned.
 Image Credit: insta_photos / Shutterstock
Image Credit: insta_photos / Shutterstock
The study
A recent paper published on the medRxiv preprint server utilized data from three national databases containing an abundance of information on Covid-19 screening (SI-DEP), vaccination (VAC-SI), and hospitalizations (SI-VIC) in France to provide additional perspectives on vaccine effectiveness and its evolution over the year 2021 in the French context.
Data from January 1st, 2021, to December 12th, 2021, was used, which allowed the authors to halt the investigation before the Omicron variant's exponential growth. This study focused on individuals aged 50 and over, a demographic that is more likely to have the most severe cases of COVID-19 and who were eligible for vaccination and then booster doses at a younger age.
The authors conducted a two-step study of vaccination effectiveness against severe forms of Covid-19, estimating vaccine effectiveness against symptomatic forms of Covid-19 and vaccine protection against hospitalization and mortality in people with symptomatic forms of Covid-19. The authors focused on the evolution of vaccine protection as a result of the combined effects of the Delta variant's appearance and the loss of immunity over time after the primary immunization scheme was completed. The authors also calculated the booster dose's impact on restoring a significant level of protection.
Between January 1st and December 12th, 2021, 8,881,107 people had been tested by RT-PCR and complained of symptoms at the moment of screening, 2,413,356 of them were over the age of 50, and 2,024,773 of them were successfully linked to vaccination data with non-missing comorbidity data. The test-negative design analysis included 432,117 COVID-19-positive cases and 864,234 controls in the sample group. At the start of 2021, a very small number of the participants were vaccinated, but by May 2021, half had received one dose, and a full dose was administered by July 2021.
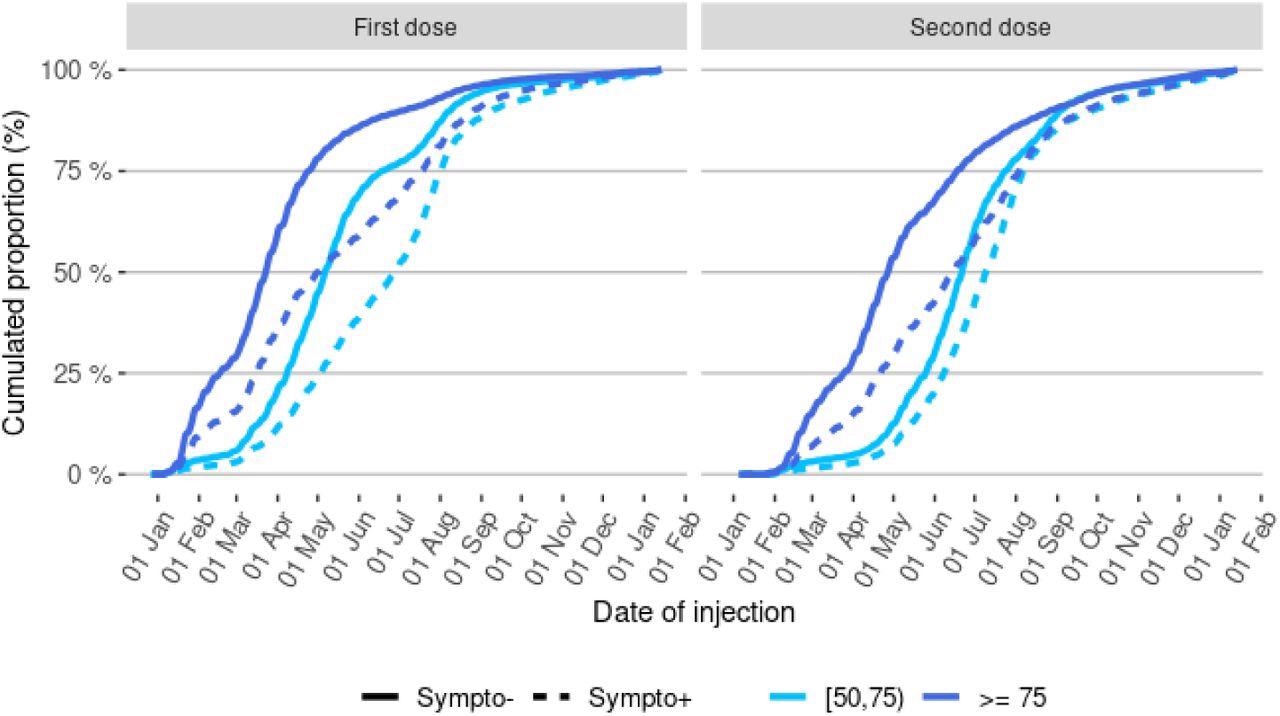
Distribution of injection dates for the first and second vaccine doses in control and cases, by age-group Abbreviations: Sympto+ (cases): symptomatic individuals with a laboratory-confirmed SARS-CoV-2 infection (cases). Sympto- (controls): individuals with symptoms non-related to SARS-CoV-2 infection.
Decreases in risk of hospitalization, admissions to ICU, and inpatient death were observed in infected symptomatic individuals as the vaccination cycle advanced. A 75% reduction in risk of hospitalization and ICU admission and a 54% reduction in risk of inpatient death was seen one month after the second dose of vaccination.
The authors then combined the estimates of vaccine efficacy against symptomatic infections with the supplementary protection offered by vaccination against severe forms of the disease in those with symptomatic infections. Following a primary vaccination cycle, the authors found that vaccine effectiveness peaked at 94 % against hospitalizations, 96% against ICU admissions, and 89% against inpatient deaths.
The vaccine's effectiveness against symptomatic infections reached its peak in the first month following the second dose in individuals aged 50 and up before plummeting to 53% within six months. The added risk reduction for ICU admissions and inpatient deaths between symptomatic patients, on the other hand, reduced only very slowly over time and stayed unchanged for hospitalizations. As a result, vaccine effectiveness against severe disease dropped at a slower and more gradual rate. Nevertheless, more than six months after the second injection, it was still about 90% against the risk of hospitalization.
Between the Alpha variant and wild-type SARS-CoV-2, there was little variation in vaccine effectiveness (91% and 92%) observed 15 days post second vaccine dose. However, the vaccine effectiveness against Beta and Delta was reduced when compared to wild-type and Alpha variants (84% and 79%). Vaccine effectiveness against symptomatic infections decreases for all variants over time. In contrast, irrespective of the variant, vaccine effectiveness against hospitalization has not been shown to decrease in the first three months. This effectiveness is four percentage points higher against the Alpha variant than against the Delta variant at the peak and for two to three months after the second dose.
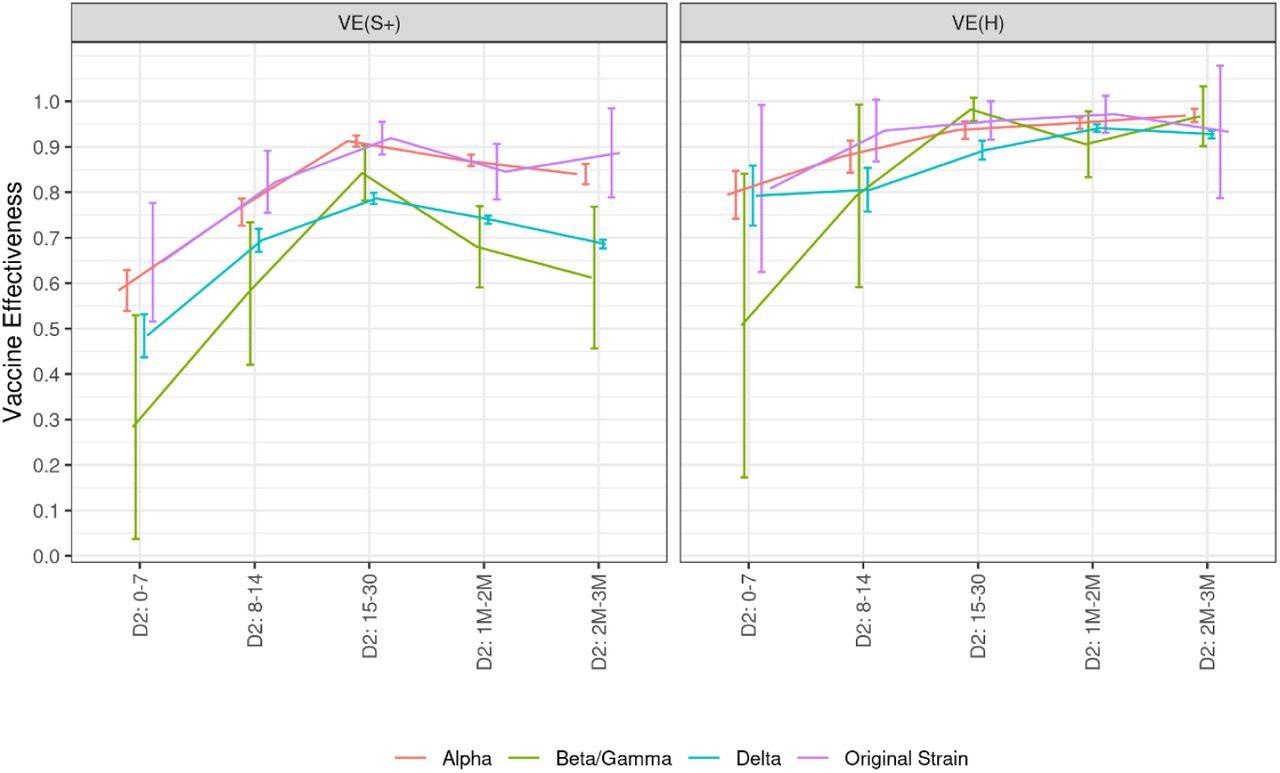
Covid-19 vaccine effectiveness against symptomatic infections and hospitalizations related to various variants of concern, according to the time elapsed since the injection of each vaccine dose, data collected from January 1st to December 12, 2021 Abbreviations: D1: first vaccine dose. D2: second vaccine dose. DB: booster dose. M: month. S+: symptomatic infection. H: hospitalization. VE: vaccine effectiveness. The numbers in the x-axis indicate the time (in days or months) elapsed since the injection of the dose of interest.
Implications
Following a full primary vaccination cycle, elevated levels of vaccine effectiveness against symptomatic infections and severe diseases were discovered. Protection was strong against every variant that circulated in France before December 2021 and the Delta variant, which did not demonstrate a strong ability to evade vaccine-induced immunity. A booster dose effectively restores the decline in effectiveness over time, which is robust against symptomatic infections but limited against severe diseases.
These results emphasize the importance of tracking vaccine effectiveness over time and optimizing booster dose vaccine uptake.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Milena Suarez Castillo, Hamid Khaoua, and Noémie Courtejoie. (2022). Vaccine effectiveness and duration of protection against symptomatic and severe Covid-19 during the first year of vaccination in France. medRxiv. https://www.medrxiv.org/content/10.1101/2022.02.17.22270791v1
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Coronavirus, Coronavirus Disease COVID-19, covid-19, Efficacy, Evolution, Healthcare, immunity, Immunization, Laboratory, Mortality, Omicron, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine
.jpg)
Written by
Colin Lightfoot
Colin graduated from the University of Chester with a B.Sc. in Biomedical Science in 2020. Since completing his undergraduate degree, he worked for NHS England as an Associate Practitioner, responsible for testing inpatients for COVID-19 on admission.
Source: Read Full Article