Tailoring collagen-based biomedical materials
Collagen is a building block that can be hierarchically assembled into diverse morphological structures that are dynamically adaptive in response to external cues. Materials scientists have limited capabilities of guiding the emergence of collagen’s hierarchical organization to recapture the richness of its biological structure and function in the lab. In a new report now published in Science Advances, Miao Lei, and a research team in materials science, medicine, and science and technology, in China and the U.S., described an electro-assembly pathway to build an intermediate molten fibril state for collagen. The intermediate state was structurally made of partially aligned and reversibly associated fibrils with limited hierarchical structure. They reversibly reconfigured the molten fibrils to offer dynamic properties such as stimuli-based stiffening, contracting, self-healing and self-shaping character and guided the molten fibrils to further assemble and recapitulate structural features of native collagen. The outcomes provide hitherto unidentified methods to tailor collagen-based biomedical materials.
Collagen as a biological building material
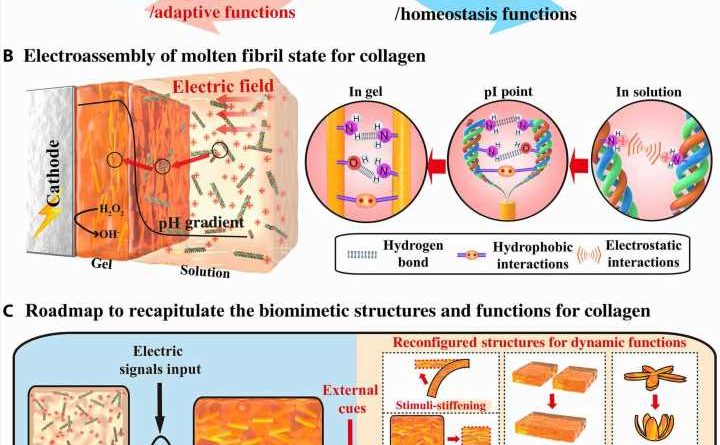
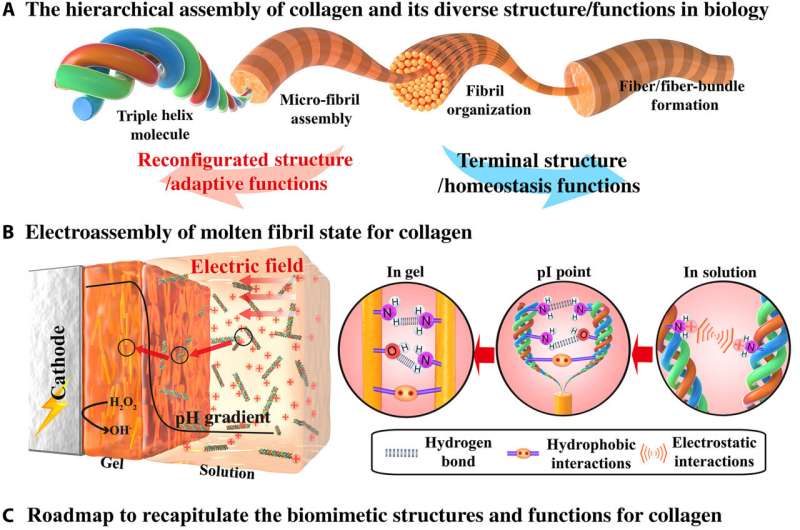
Structural proteins form important building blocks in biology with a variety of molecular interactions to cue their hierarchical assembly into complex morphological structures to regulate functional properties. Collagen is a classic example where triple helix molecules can be used to organize across a hierarchy of lengths and scales. Such assemblies can be connected via reversible interactions for structural reconfigurations and adaptive functional properties. For example, interactions seen with sea cucumbers describe dynamic cross-linking of collagen fibrils to reversibly tune their biomechanics and avoid predation. Such dynamics can also facilitate wound healing and remodeling via cell migration and self-organization. Examples of these structures in physiological environments include cross-linked collagen microfibrils of the transparent cornea, collagen fibrils of bone and teeth, skin and tough tendons. In this work, Lei et al. described the electro-assembly of an intermediate molten fibril state for collagen with a partially aligned fibril structure, but with limited hierarchical organization. They expect the electro-assembly of intermediate molten fibril states of collagen to provide previously unidentified opportunities to form collagen-based biomedical materials that mimic and incorporate structural properties of native collagen and collagen materials.
Electro-assembly of collagen with a molten fibril state
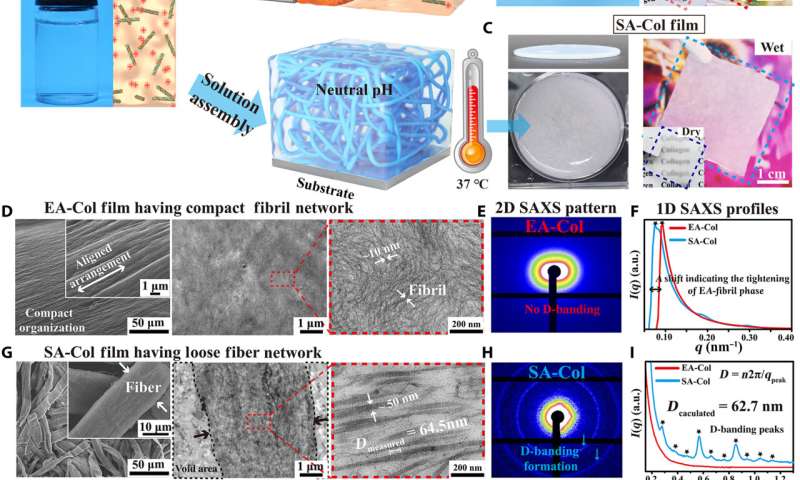
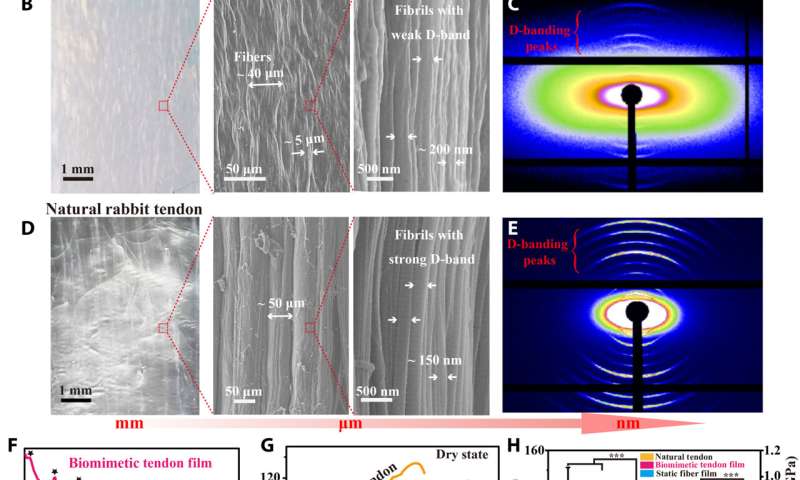
Lei et al. first obtained acid-solubilized collagen from porcine skin to form a transparent molecular solution in acetic acid. They then induced the collagen solutions to self-assemble onto a titanium foil by imposing a cathodic voltage, when the pH of the solution was elevated. For comparison, the team prepared collagen films of approximately the same thickness via a conventional method. Such collagen hydrogel films maintained an opaque milky white appearance, designated as SA-Col, and remained semi-transparent. The high transparency of the electro-assembled collagen (designated EA-Col) could be stabilized via chemical crosslinking with benefits for biomedical applications including corneal implants that require long-term transparency. Lei et al. used scanning electron microscopy and transmission electron microscopy to show the microstructure of EA-Col films with dense organization and an aligned fibrous filamentous surface. They noted the organization of fibrils with a diameter of 10 nm. Then using nanostructure analysis, they performed synchrotron small-angle X-ray scattering to highlight the distinct, partially aligned fibril structure of the EA-Col network, in response to the imposed electric field.
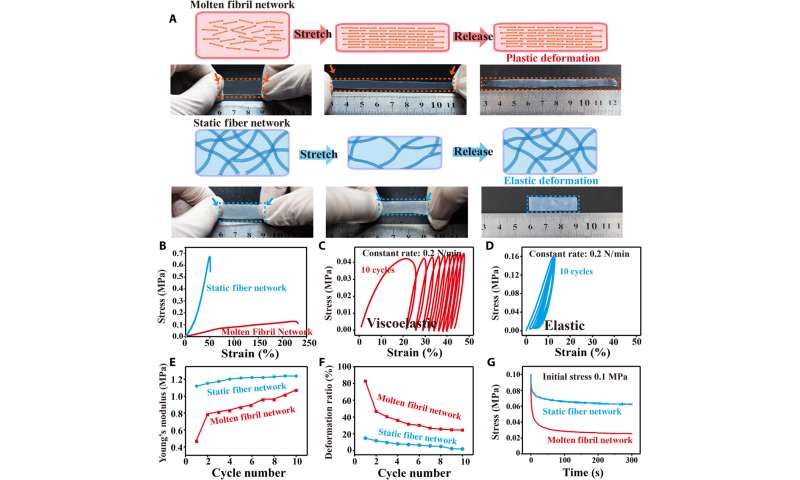
Dynamic adaptability of molten fibril to mechanical forces
The scientists next studied the dynamic stability of molten fibrils to mechanical forces. They accomplished this by obtaining representative stress-strain curves to show the weakness of the molten fibril network, which underwent large deformation and gradual fracture. In contrast, the static fiber network had higher modulus, less deformation and underwent brittle fracture during stretch-release experiments. To further investigate the mechanical characteristics of molten fibrils, Lei et al. performed multicycle dynamic tensile loading measurements to note an increase in Young’s modulus during each consecutive loading cycle. They then investigated the mechanical variability of the collagen tissue structure to realize key physiological functions. For example, in nature, sea cucumbers can rapidly stiffen their connective networks to avoid predation by relying on the reversible regulation of interactions among adjacent collagen fibrils. Bioinspired by such interactions, Lei et al. incorporated Hofmeister ions that can influence hydrophobic (water-repelling) interactions to strengthen internal connections of the collagen fibril network. The collagen’s adaptive molten fibril state was highly responsive to the ions to adjust physical crosslinking and facilitate mechanical properties of molten fibril networks.
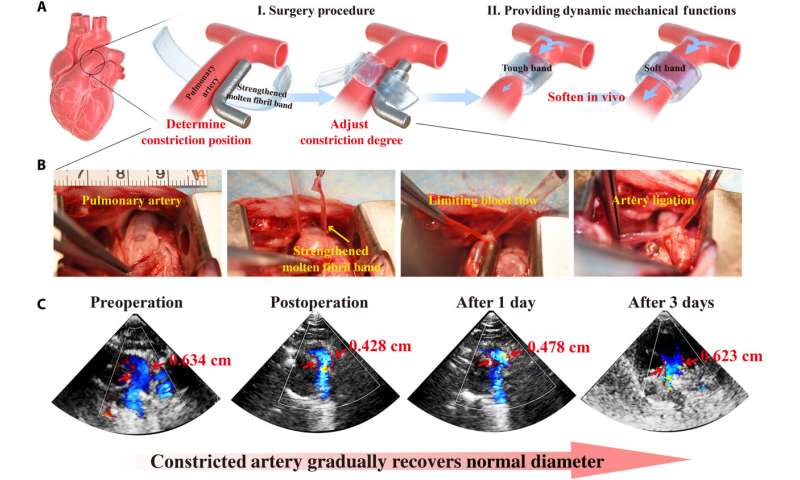
Dynamic mechanical functions of the fibril networks in vivo and further experiments
Since the strengthening process of molten fibril networks by the Hofmeister effect is reversible, the team sought to develop biomedical concepts to highlight the adjustable and emergent properties of the construct, to thereby meet design objectives that can mechanically change with time. For example, during some surgical interventions, surgeons implant a band around an artery to constrict blood flow and protect a vulnerable downstream site from hypertension. While immediately after surgery this band should be strong, with time it should relax to allow greater blood flow. The ideal material to meet this medical requirement would therefore have mechanical properties that dynamically relax under conditions in vivo. A molten fibril network strengthened by a Hoffmeister salt could provide adequate strength to relax as the salt leached from the network, and after a period of time, the construct will be resorbed via preliminary biodegradation. Thereafter, the team tested the possibility of reconfiguring the structure and properties of molten fibril networks by regulating electrostatic interactions. Followed by experiments to highlight stimuli-contracting and self-healing properties of the material bioinspired by Cephalopods to create 3D complex shapes and form promising candidates for applications as bio-actuators, soft robots, and other intelligent biomimetic devices.
Outlook
Source: Read Full Article