Novavax Seeks FDA Authorization for COVID Vaccine
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
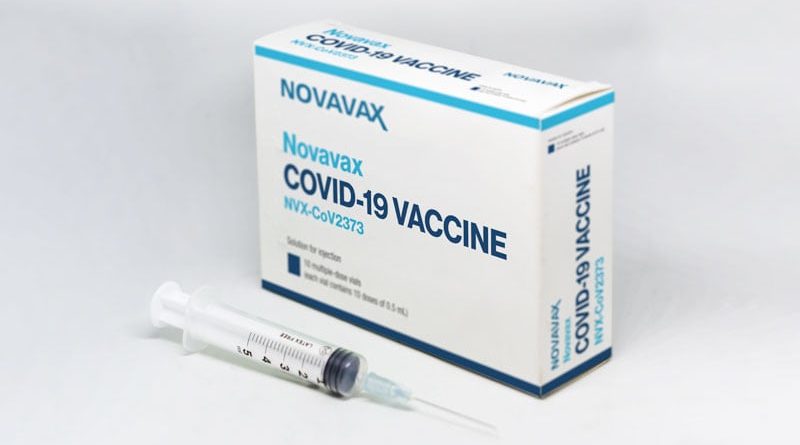
Novavax announced Monday that it has formally submitted a request to the FDA for emergency use authorization of its COVID-19 vaccine for ages 18 and older.
The request includes results from two large clinical trials that showed an overall efficacy of about 90% and a “reassuring safety profile,” the company said.
“We believe our vaccine offers a differentiated option built on a well-understood protein-based vaccine platform that can be an alternative to the portfolio of available vaccines to help fight the COVID-19 pandemic,” Stanley Erck, the president and CEO of Novavax, said in the statement.
Known as NVX-CoV2373, the vaccine is protein-based and engineered from the genes of the first strain of SARS-CoV-2, the coronavirus. With technology like that of the flu vaccine, it uses antigen from the coronavirus spike protein to trigger immunity. Two doses are given 21 days apart.
Novavax announced in June 2021 that the vaccine had an overall efficacy of 90% in late-stage clinical trials in the US and Mexico. The trials took place before the Omicron variant was dominant in the US, according to CNN. In December, the company said it completed its final submission package for the FDA application.
The two clinical trials enrolled 30,000 people in the US and Mexico and 15,000 in the UK. In both trials, serious and severe adverse events were low, and the most common bad reactions included headache, nausea, vomiting, muscle pain, joint pain, fatigue, and pain where the shot was given.
The vaccine can be stored at normal refrigeration temperatures between 2 and 8 degrees Celsius, or about 35 to 46 degrees Fahrenheit, and it has a shelf life of about 9 months. Erck told CNN in November that the company could ship the first 100 million doses of the vaccine once the FDA gives authorization.
In November, Indonesia was the first country to grant emergency use authorization for Novavax’s vaccine, CNN reported. Since then, regulators in Australia, the European Union, India, the Philippines, and South Korea have granted authorization.
Novavax has also applied for authorization in other countries, according to the news outlet, including Canada, the UK, and New Zealand. If authorized by the FDA, the Novavax vaccine would be the first protein-based COVID-19 vaccine in the US.
Novavax is also studying its vaccine in ages 12-17 and testing a third dose as a booster.
For more news, follow Medscape on Facebook, Twitter, Instagram, and YouTube.
Source: Read Full Article