Lung Atlas Maps Cell Changes in Idiopathic Pulmonary Fibrosis
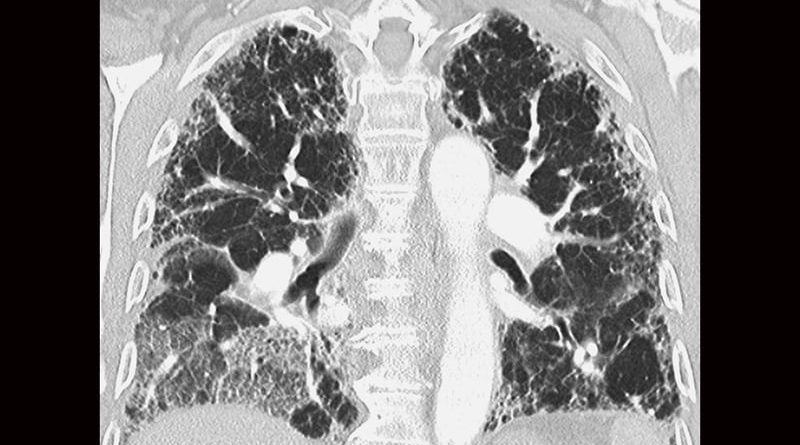
An online atlas of lung cells affected by idiopathic pulmonary fibrosis (IPF) may help researchers find ways to create new therapies to treat this rare disease, one affecting about 100,000 Americans. At present, scientists are uncertain as to what causes the hardening and scarring of lung tissues that lead to increasingly labored breathing in severe IPF cases, stated Louis Vuga, MD, PhD, program director, Division of Lung Diseases, National Heart, Lung, Blood Institute (NHLBI), in an interview. The Idiopathic Pulmonary Fibrosis Cell Atlas maps IPF cells and may yield important disease process insights.
The occasion for attention to the cell mapping project is the NHLBI’s February 28th Rare Diseases Day, which is being held to raise awareness of research conducted in support of individuals living with rare or uncommon heart, lung, and blood conditions. The designation “rare,” attributed to conditions that occur in fewer than 200,000 people, encompasses 7000 diseases that affect about 1 in every 12 Americans. Around 50,000 new cases of IPF are diagnosed each year in the United States. While a history of smoking and some genetic profiles have been shown to predispose individuals, a direct cause of IPF has not been identified.
The NHBLI-funded project, conducted by a research team from Yale University and Baylor College of Medicine, was preceded just a year or so earlier by another NHBLI-funded mapping project, the Normal Aging Lung Cell Atlas, led by Naftali Kaminski, MD, professor of medicine at Yale. The intention of the project was to generate a compendium of the dynamic cell-specific changes in mRNA, microRNA, and epigenetic marks that occur during normal lung aging, with a focus on single cells and the alveolar microenvironment. Given that IPF is more common among adults aged 50–70 years, it serves well as a research reference for the Idiopahtic Pulmonary Fibrosis Cell Atlas.
To uncover the cause of the chronic thickening and stiffening of tissues surrounding the alveoli associated with IPF, researchers analyzed a total of 330,000 cells from 79 human lungs, detailing changes in each cell through single-cell RNA sequencing. Their investigations into IPF have already pointed to a definite shift in lung cells responsible for respiration. Kaminski told YaleNews (July 8, 2020), “The cells in the distal lung, in the air sacs, or alveoli, that specialize in respiration seem to be replaced by cells from the airways that specialize in air delivery.” He described the changes as an attempt at self-repair. These “misplaced” cells were found to be prominent in the alveoli lining.
A newly discovered cell type, termed by the researchers “aberrant basaloid cells,” is involved in scarring and appears in a region of the lung long thought to be lung fibrosis’s leading edge. The team also observed distinct alterations in blood vessel cells that are usually found only in airways. The general picture, that IPF causes cell movements and the arising of new cell populations, is consistent with a theory that Kaminski proposed more than a decade back, attributing the lung fibrosis scarring to “out-of-context lung development” — that is, an attempt of the lung to replace what the disease has destroyed.
For more news, follow Medscape on Facebook, Twitter, Instagram, and YouTube.
Source: Read Full Article