Immunotherapy combined with targeted therapy for colorectal cancer yields promising outcomes for patients
A new study that used insights from the lab to drive a clinical trial for patients with a difficult-to-treat form of colorectal cancer improved patients’ response to treatment and has yielded key insights with broad relevance to other forms of cancer.
Led by investigators from the Mass General Cancer Center, a member of Mass General Brigham, the proof-of-concept, single-arm phase 2 clinical trial included 37 patients with BRAF V600 mutations, which are found in about 10 percent of colorectal cancers. The work represents the first clinical trial combining immunotherapy and targeted therapy for this patient population.
In a paper published in Nature Medicine, the team reports a long-lasting response among patients who responded to the combined treatment and reveals how a targeted therapy may cooperate with an immunotherapy for better results.
“Immunotherapy and targeted therapy represent two of the biggest breakthroughs in cancer treatment in the last decade,” said co-corresponding author Ryan Corcoran, MD, Ph.D., Director of the Gastrointestinal Cancer Center Program and physician-investigator in the Mass General Cancer Center. “By combining these two approaches, we saw a dramatic increase in patients who responded to treatment and unprecedented durability, with 18 percent of patients staying in the trial for a year or more.”
“Our findings suggest that there is tremendous potential for these two therapies to cooperate when given together,” said co-corresponding author Nir Hacohen, Ph.D., director of the MGH Center for Cancer Immunotherapy. “This merits further clinical investigation and pre-clinical experiments to determine the best targeted approach to increase immune reactivity against colorectal cancer with mutated BRAF.”
BRAF mutations occur in many kinds of cancers, most commonly in melanoma. BRAF is a component of the MAPK signaling pathway—a chain of proteins that, when disrupted by genetic mutations, can lead to unchecked cell growth. Several drugs for treating cancer have been developed to target components of the pathway.
In melanoma, BRAF inhibitors have been highly effective, with a majority of patients showing strong initial responses to the drug. But response to BRAF inhibitors occurs in less than 5 percent of patients with colorectal cancer in which BRAF has been mutated. Immunotherapy—which has also been extremely effective against some types of cancer—has generally not worked well against colorectal cancers (with the exception of approximately 4 percent of cancers with an unusual feature known as microsatellite instability).
Corcoran, Hacohen and colleagues drew inspiration for the clinical trial from observations seen in preclinical models. In these models, investigators saw signs that inhibitors targeting the MAPK pathway, including BRAF, might enhance the immune system’s response. Based on these observations, the team investigated whether combining a therapy targeting BRAF could enhance the effectiveness of the immunotherapy.
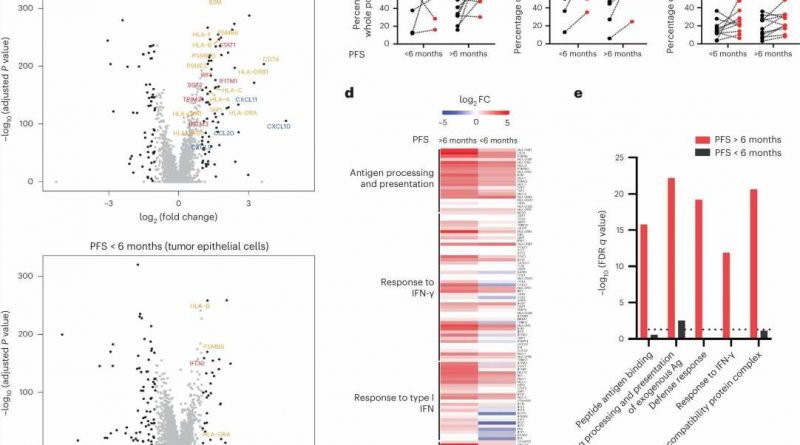
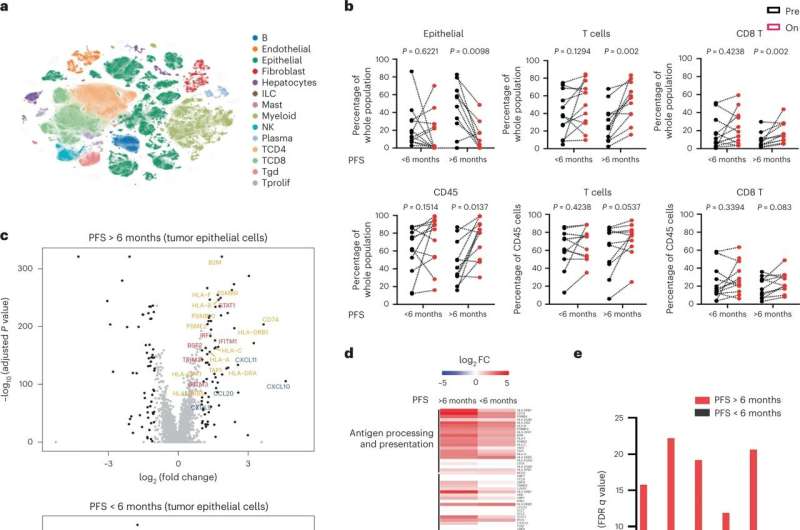
To investigate this potential cooperativity, the investigators analyzed samples collected from 71 patients before and during an earlier clinical study in which patients received BRAF targeted therapy only. Using single-cell RNA sequencing, the team looked for molecular changes that occurred due to treatment. Based on what they saw, they initiated a clinical trial in which patients with a specific BRAF mutation known as V600E received the BRAF inhibitor dabrafenib, the MEK inhibitor trametinib, and the immunotherapeutic drug spartalizumab.
The study met its primary endpoint, with a confirmed response rate of 24.3 percent of patients (compared to a response rate of only 7 percent in a prior trial where patients were treated with the same targeted therapies alone). The team also saw promising results in one of the trial’s secondary endpoints: durability.
When BRAF or MEK inhibitors have been given to colorectal cancer patients with BRAF mutations in the past, even among those who responded, the clinical benefit has been short-lived. But the combined treatment increased durability with a median progression-free survival of 5 months (versus 3.5 months with BRAF/MEK alone), with 57 percent of patients remaining on treatment for more than 6 months and 18 percent for more than a year.
The team also performed single-cell RNA sequencing on samples collected before and on day-15 of combined treatment. For patients who had better clinical outcomes, investigators saw an increase in tumor cell-intrinsic immune programs and more complete MAPK inhibition. This suggested that improving MAPK inhibition, perhaps by focusing on other treatment targets in the pathway, may drive a greater immune response and improve treatment overall. Other clinical trials are currently underway to further explore this.
Corcoran says that the implications of the work may go well beyond colorectal cancer.
“For almost every type of cancer, a large percentage of tumors will harbor mutations in the MAPK pathway,” he said. “Our work suggests that combining other treatments that target this pathway with an immunotherapy could lead to a cooperative, enhanced immune response that may improve outcomes for patients.”
More information:
Nir Hacohen, Combined PD-1, BRAF and MEK inhibition in BRAFV600E colorectal cancer: a phase 2 trial, Nature Medicine (2023). DOI: 10.1038/s41591-022-02181-8. www.nature.com/articles/s41591-022-02181-8
Journal information:
Nature Medicine
Source: Read Full Article