Gene linked to glioblastoma stem cell self-renewal and immunosuppression
Northwestern Medicine scientists have identified how one gene connects glioblastoma stem cell self-renewal to microglia immunosuppression in glioblastoma, according to a new study published in Nature Immunology.
Glioblastoma, one of the most complex and treatment-resistant cancers, has a five-year survival rate of just 6.9%, according to the National Brain Tumor Society. The average length of survival is estimated to be only eight months, a figure that has barely improved since glioblastoma was first identified in scientific literature in the 1920s.
Glioblastoma is particularly difficult to treat because tumors are made up of a variety of different cells that can induce resistance to conventional therapy and immunotherapy, including self-renewing glioblastoma stem cells (GSCs) and immunosuppressive microglia, resident immune cells of the brain that have been reprogrammed in the tumor microenvironment, said Peiwen Chen, Ph.D., assistant professor of Neurological Surgery and senior author of the study.
“GSC stemness and microglia immunosuppression are two key hallmarks of glioblastoma,” said Chen, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “Before, we didn’t really understand the mechanisms behind how these two have a symbiotic interaction with each other.”
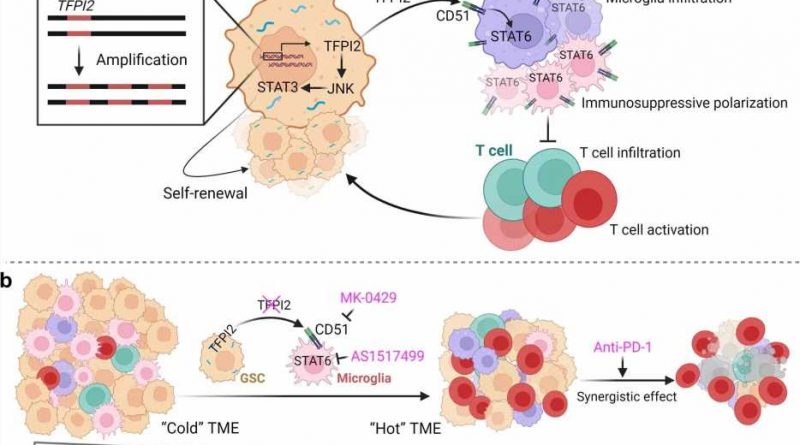
In the study, investigators first analyzed data from The Cancer Genome Atlas Glioblastoma dataset and upon comparing survival data and genes over-expressed in tumors that correlated with tumor stemness, or the ability of the GSCs to self-renew and proliferate, they found that the gene TFPI2 is amplified in a subset of glioblastoma tumors.
Study investigators then knocked out TFPI2 in glioblastoma stem cells taken from both mice and humans and observed a drop in tumor cell self-renewal and proliferation.
In living mice with glioblastoma, deletion of TFPI2 both inhibited tumor growth and lengthened survival, according to the study.
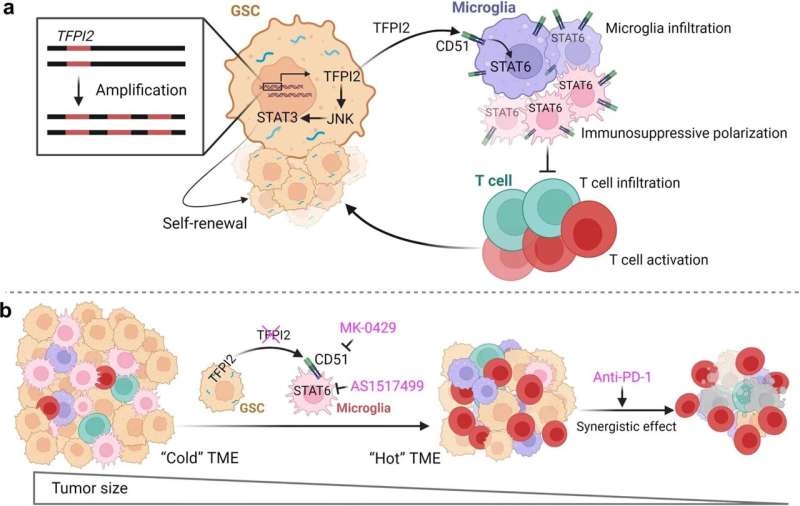
Upon further inspection using RNA sequencing on control and TFPI2-depleted glioblastoma stem cells, scientists found that TFPI2 proteins mediate stemness by activating a handful of related pathways known to promote cancer stem cell maintenance in many types of cancer. Additionally, GSC-secreted TFPI2 triggers the infiltration of microglia and causes them to become immunosuppressive in the tumor microenvironment, according to the study.
“We found that the TFPI2 is amplified in glioblastoma tumors and/or over-expressed in GSCs where it can promote GSC self-renewal through activating the JNK-STAT3 pathway,” Chen said. “And on the other hand, we found that TFPI2 can be secreted from GSCs to trigger microglia infiltration into the tumor microenvironment and also promote microglia immunosuppressive polarization via activation of its receptor CD51 and the downstream signaling STAT6 in microglia.”
Inhibition of the signaling pathway impairs tumor growth, activates T-cells and synergizes with therapy in glioblastoma mouse models, according to the study.
Taken together, the findings identify TFPI2 as a major player in regulating GSC stemness and microglia immunosuppression and provide a new potential target for treating the aggressive tumors.
“We have linked the two hallmarks of glioblastoma and really understand the mechanism for this process, and we also identified therapeutic targets to block the GSC-microglia symbiosis,” Chen said. “Now we can really think about translational approaches for how to bring this into a clinical setting.”
Chen and his collaborators have already filed a patent for the inhibitors used in the study and will begin designing a clinical trial to further validate the treatment, he said.
More information:
Lizhi Pang et al, Kunitz-type protease inhibitor TFPI2 remodels stemness and immunosuppressive tumor microenvironment in glioblastoma, Nature Immunology (2023). DOI: 10.1038/s41590-023-01605-y
Journal information:
Nature Immunology
Source: Read Full Article