Emergence of SARS-CoV-2 immune escape variants in an immunocompromised patient
A German case study recently published in the journal Nature Communications has characterized a long-term severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection dynamics in an immunosuppressed kidney transplant recipient and provided evidence for the evolution of SARS-CoV-2 into potentially harmful immune escape mutants during persistent infections.
Such escape mutants likely serve as the initial seed for emerging variants with enhanced epidemic potential, especially if they overcome impaired viral fitness by further adaptation.
.jpg)
Quest to figure out the origin of virus variants
Continuously emerging SARS-CoV-2 variants pose a global threat due to their increased transmissibility and resistance to neutralizing antibodies. The origin of these virus variants, however, remains unclear.
Reports suggest long-term-infected immunocompromised individuals could be a possible source, as they may allow unhindered viral replication and adaption to the host.
Study involved SARS-CoV-2 positive kidney transplant recipients on immunosuppressants
Immunosuppressed individuals are expected to have an increased risk of developing severe coronavirus disease 19 (COVID-19) and to suffer from long-term persistent infection with prolonged viral shedding. Therefore, such patients are being closely monitored in Germany during the prevailing pandemic.
The present case study involved one such patient who received a renal transplantation in March 2020, was on immunosuppressant drugs from March until the end of September to prevent the risk of organ rejection, and tested positive for SARS-CoV-2 a few days later after transplantation. The infection progressed to persistence, as evident from a positive RT-qPCR on day 140 (from initial positive testing for SARS-CoV-2/day 0). For control purposes, Remdesivir treatment was started for 10 days. Subsequent negative RT-qPCR and no virus isolation until day 189 suggested the resolution of infection.
SARS-CoV-2 genome mutated in later weeks of persistent infection
Phylogenetic analysis demonstrated clustering of viral genome sequences, isolated in initial weeks, with the strains circulating in March in Germany. From day 42 onwards, the acquisition of several mutations occurred. Apart from low-frequency mutations, some mutations accumulated over time, indicative for the selection of distinct variants.
Notably, the day 105 specimen demonstrated in-frame deletions and non-synonymous substitutions in the N-terminal domain (NTD) and the receptor-binding motif (RBM), respectively. Furthermore, the two amino acid deletions in the NTD were associated with specific single amino acid substitutions in the RBM: del141-144 with subF490L and del244-247 with subE484G, suggesting the emergence of at least two variants.
The team suggests that this interlinked coevolution may have been favored by the necessity to preserve the spike protein's functionality or stability and maintain viral fitness. Both deletions, which are located in two adjacent flexible loops of the NTD, are suggested to affect the conformation of this subdomain, a target of neutralizing antibodies.
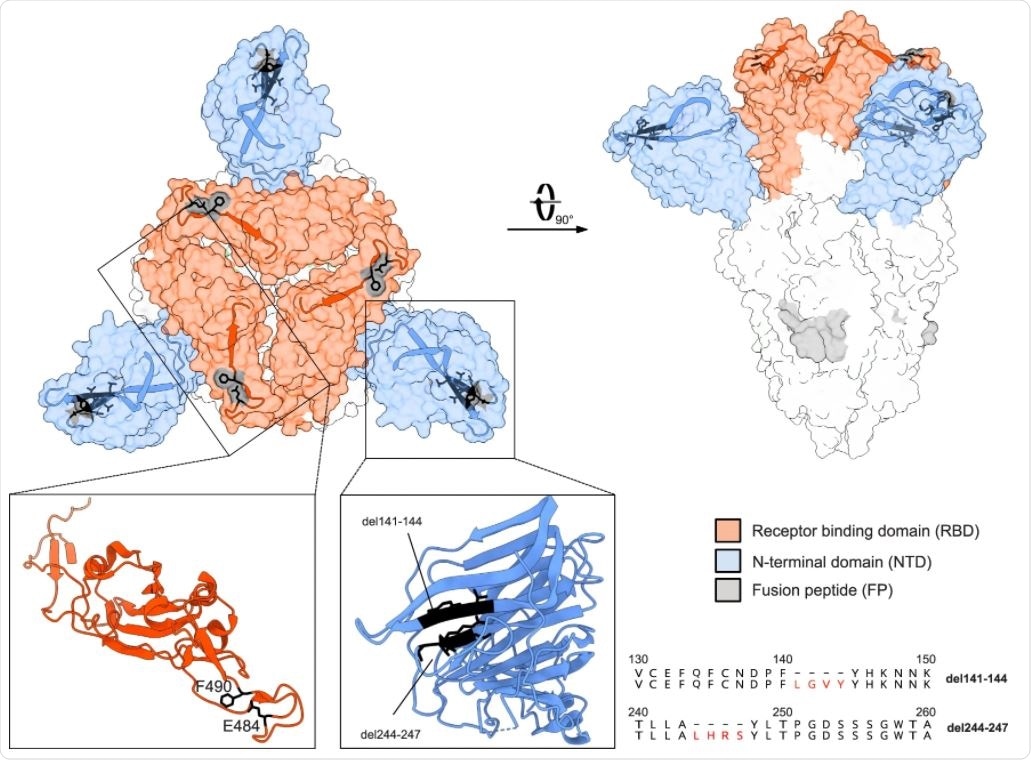
In addition, the two amino acid substitutions (E484G and F490L) may lead to subtle conformational changes in the RBD, which is the main target of neutralizing antibodies and a known hotspot for mutations conferring escape from neutralizing antibodies.
These mutations in SARS-CoV-2 variants found in an immunosuppressed patient are shared with the escape variants of concern from the UK, South Africa, and Brazil.
The team thus speculates that prolonged viral replication in immunosuppressed patients can lead to the emergence of new immune escape variants, such as the alpha, B.1.1.7, the beta, B.1.351, and the gamma variants, P.1 that all share mutations in the same regions of the spike protein as the escape variants described in this study.
SARS-CoV-2 mutations in later weeks of persistent infection did not reduce virulence
To confirm the growth phenotype of day 14 and day 105 virus isolates in vivo, K18-hACE2 mice- encoding the human SARS-CoV-2 receptor, angiotensin-converting enzyme type 2 (ACE2) was intranasally infected with both the viral isolates.
Severe disease symptoms and weight loss in mice strongly suggested that the mutations in the spike gene and other regions of the day 105 genome caused no decline in viral fitness.
SARS-CoV-2 mutants show escape from neutralizing antibodies
Nucleoprotein (N)-specific antibodies were detected early in infection just after the first positive RT-qPCR result and afterward surged rapidly. In contrast, IgG antibody levels specific for SARS-CoV-2 spike protein (S1) constantly oscillated around the threshold value between days 40 and 123. High levels of S1-specific IgG were detected later around day 140 and persisted at least until day 175.
Only sera collected from the patient after day 123 showed SARS-CoV-2 neutralizing activity in plaque reduction assay, which coincided with the increase of S-specific IgG between days 123 and 140.
Antisera of convalescent COVID-19 patients and of individuals previously vaccinated with the BNT162b2 mRNA vaccine (Pfizer/BioNTech) showed higher neutralizing activity against the day 14 isolate as compared to the day 105 isolate. This suggested that the mutations in the day 105 spike protein caused an escape from neutralizing antibodies.
In a virus pseudotype system, high neutralization titer 50 (NT50) values were observed with the d14 spike protein, compared to the pseudotype virus bearing the d105 spike protein as indicated by an 8.3-fold reduced NT50 value. Additionally, compared to pseudotype virus displaying the parental d14 spike protein, viruses pseudotyped with amino acid deletions del141-144 or del244-247 (but not any substitutions) were significantly less neutralized by a COVID-19 convalescent serum.
A different pattern was observed with immune serum from a person vaccinated with an mRNA-based SARS-CoV-2 vaccine. The virus pseudotyped with the E484G mutant spike was less neutralized than the virus bearing the parental day 14 spike protein. Furthermore, pseudotype virus displaying spike protein containing both the E484G substitution and the del141-144 deletion showed a reduced NT50 value compared to pseudotype viruses containing either E484G or del141-144 alone, suggesting a synergistic effect of these two mutations.
In summary, the amino acid changes del141-144 and del244-247, both located in the NTD, and E484G in the RBD selected during viral persistence, were all found to affect crucial antigenic regions as they allow the escape of SARS-CoV-2 from the immune responses.
"Because these globally emerging viruses show a clear escape from vaccine-induced humoral immunity, our findings might be important for the redesign of future vaccines by introducing combinations of mutations into the spike gene that might broaden the specificity of the antiviral immune response," the team concludes.
- Panning M, and Kochs G, et al. (2021) Within-host evolution of SARS-CoV-2 in an immunosuppressed COVID-19 patient as a source of immune escape variants. Nature Communications 12, 6405. doi: https://doi.org/10.1038/s41467-021-26602-3, https://www.nature.com/articles/s41467-021-26602-3.
Posted in: Medical Research News | Medical Condition News | Disease/Infection News
Tags: ACE2, Amino Acid, Angiotensin, Antibodies, Antibody, Assay, Coronavirus, Coronavirus Disease COVID-19, Drugs, Enzyme, Evolution, Frequency, Gene, Genome, Immune Response, immunity, in vivo, Kidney, Kidney Transplant, Pandemic, Phenotype, Protein, Receptor, Remdesivir, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Transplant, Vaccine, Virus, Weight Loss

Written by
Namita Mitra
After earning a bachelor’s degree in Veterinary Sciences and Animal Health (BVSc) in 2013, Namita went on to pursue a Master of Veterinary Microbiology from GADVASU, India. Her Master’s research on the molecular and histopathological diagnosis of avian oncogenic viruses in poultry brought her two national awards. In 2013, she was conferred a doctoral degree in Animal Biotechnology that concluded with her research findings on expression profiling of apoptosis-associated genes in canine mammary tumors. Right after her graduation, Namita worked as Assistant Professor of Animal Biotechnology and taught the courses of Animal Cell Culture, Animal Genetic Engineering, and Molecular Immunology.
Source: Read Full Article



