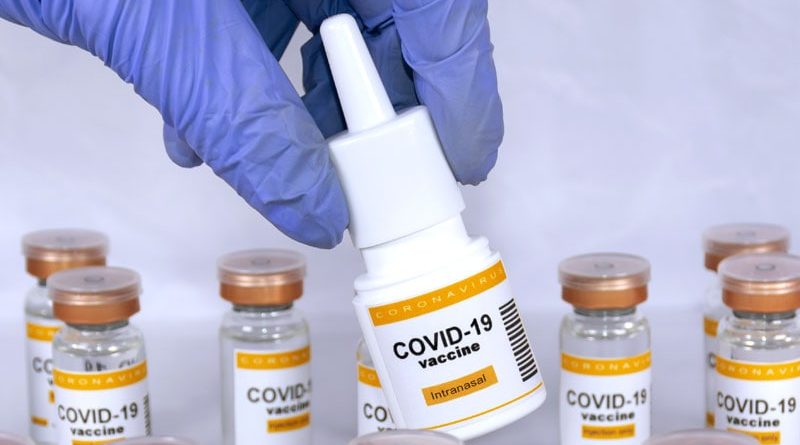
Clinical Trials of Nasal COVID Vaccine to Start This Year
TOURS, France — A protein-based vaccine for COVID-19 administered through the nose has been developed by the BioMAP team at the French National Research Institute for Agriculture, Food, and Environment (INRAE)–University of Tours Infectious Diseases and Public Health (ISP) joint research unit. This vaccine can trigger an immune response at the systemic level, similar to traditional intramuscular vaccines, as well as at the mucosal level.
“Instillation in the nose induces an immune response in the nasal cavities. Because of this, we’re able to stop the virus very early on. This means it will no longer be able to multiply and infect the people around you,” explained Isabelle Dimier-Poisson, PhD, an expert in the immunology of infectious diseases at the University of Tours, and incoming general and research manager at LoValTech, a start-up that will lead development of the vaccine formulation. “Intramuscular vaccines protect against severe forms of COVID. However, if you catch the virus, you could infect others. The intranasal vaccine does away with this problem,” she explained.
Like traditional vaccines, the intranasal vaccine is effective against serious forms of COVID-19. And in addition to being able to stop the virus early in the nasal cavities, it has several other advantages: “All of the current COVID-19 vaccines on the market are based on the spike protein, which then mutated, rendering those vaccines less effective. Our vaccine contains not only the spike protein, but other proteins that do not mutate and are found in all variants. The vaccine can be stored at 39 °F (~4 °C), but survives at room temperature, which makes it ideal for countries where meeting cold-chain requirements can be a challenge,” said Dimier-Poisson. Another plus is that administration of this vaccine is noninvasive, which can make the immunization procedure less stressful for people, especially children.
A Very Strong Systemic and Mucosal Immune Response
The team filed a patent application for the intranasal vaccine in September 2021. The researchers are currently completing preclinical trials, and results so far have been promising. “A very strong systemic and nasal mucosal immune response was induced” when researchers delivered two nasal immunizations, 3 weeks apart, in a mouse model, she reported.
But can the vaccine protect against serious forms of COVID-19? Using mice that had been genetically modified to be susceptible to SARS-CoV-2, the researchers administered two doses of the vaccine one group; the mice in the control group received nothing. When they then infected the mice with the virus, all the vaccinated mice survived and developed no symptoms (no respiratory distress, no weight loss, etc.), whereas the mice in the control group all exhibited symptoms of respiratory distress.
To assess how well the vaccine prevented transmission of the virus, the researchers administered two doses of the vaccine, 3 weeks apart, to hamsters. “The hamsters showed no viral load in the lungs,” said Dimier-Poisson.
To determine the vaccine’s effectiveness against COVID-19 variants, the team conducted in vitro and in vivo studies. “We were able to show the vaccine’s efficacy against Beta and Delta. We’re currently finalizing tests on Omicron,” she said.
So far there have been no adverse effects. “This is a protein-based vaccine without adjuvant. It’s totally safe. We fully expect that it’ll be used for children and immunocompromised patients,” Dimier-Poisson explained.
The production phase of Good Manufacturing Practice got underway thanks to efforts that raised close to €3 million (~US$3.4 million), which includes €1.5 million (~US$1.7 million) from the Ministry of Higher Education, Research, and Innovation (MESRI), and €900,000 (US$1.3 million) from ANRS | Emerging Infectious Diseases.
“We’ve embarked on a creative biotech process to continue developing the vaccine,” said Dimier-Poisson, who noted that “we’ll still need a substantial amount of money to carry on with that development.” The team plans to start the first clinical trial before the end of 2022, and hopes that the vaccine will be on the market by 2024.
“Even if the pandemic ends, the virus will still be around as an epidemic. People who got an intramuscular vaccine will be able to take our nasal vaccine to prevent them from transmitting the virus. Our vaccine will also be directed at the many people around the world who are not yet immunized. We’re committed to making a vaccine available to these populations at cost,” stated Dimier-Poisson, who believes that “the vaccine will be of interest to everyone, whether as an initial immunization or as a booster.”
Not many vaccines are administered through the nose; the World Health Organization lists only six. “We’re the only ones in France who are developing this type of vaccine,” said Dimier-Poisson. “It’s a message of hope that we’ll all get back to the way life was before COVID.”
This article originally appeared in the French edition of Medscape.
Follow Medscape on Facebook, Twitter, Instagram, and YouTube
Source: Read Full Article