Cerebrovascular impairment persists months after severe acute COVID-19, UK study
Researchers in the United Kingdom have conducted an observational cohort study on hospitalized patients and have found that the degree of severity of acute coronavirus disease 2019 (COVID-19) predicts the occurrence of chronic cerebrovascular impairment. The study further suggests that the levels of cerebrovascular impairment are associated with worse cognitive, mental, and physical well-being.
The cerebrovascular abnormality was found to be localized across lateral frontotemporoparietal regions of the brain, and its genetic signature was responsible for shaping the composition of cell types and metabolism.
A pre-print version of the research paper is available on the medRxiv* server while the article undergoes peer review.
 Study: Hospitalisation for COVID-19 predicts long-lasting cerebrovascular impairment: A prospective observational cohort study. Image Credit: SvedOliver / Shutterstock
Study: Hospitalisation for COVID-19 predicts long-lasting cerebrovascular impairment: A prospective observational cohort study. Image Credit: SvedOliver / Shutterstock
Background
COVID-19, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has multiple system effects, including neurological and vascular consequences. It is common for COVID-19 patients to suffer from neurological sequelae ranging from mild dizziness, headaches, and anosmia to severe encephalitis, stroke, and delirium.
A poor cerebrovascular reactivity, which is the constricting or dilating capacity of cerebral vessels of the brain in response to physiological conditions in order to regulate regional blood flow, may increase the risk of neurodegeneration. Likewise, the cerebrovascular consequences of COVID-19 may also influence brain health. While this acute pathophysiology is detectable using neuroimaging, the persistence of long-term effects of cerebrovascular disability remains unknown.
What did the researchers do?
The scientists assessed cerebrovascular health in 45 hospitalized patients, in relation to COVID-19 severity. The resting-state fluctuation amplitudes (RSFA), which is the blood oxygenation level-dependent (BOLD) signal variability at rest, were measured via functional magnetic resonance imaging (MRI). RSFA is a robust, scalable, safe, and easy-to-apply method of determining cerebrovascular responsiveness in clinical cohorts. The study involved comparison with age and sex-matched 42 controls.
Clinical data were obtained from electronic medical records and cardiorespiratory and neurological assessments at follow-up visits at least 6 weeks following symptom onset.
The severity of acute disease was quantified by the COVID-19 World Health Organization (WHO) Progression Scale and the blood biomarkers (including hematological, inflammatory, immunological, hepatic and coagulation biomarkers) in patients hospitalized with COVID-19. The WHO 11-point COVID-19 Progression scale was used to score disease severity from 0 (non-infected) to 10 (dead).
Cardiorespiratory measurements at least 12 weeks after discharge from initial hospitalization with COVID-19 were also collected. Quality of life, cognition and mental health were assessed using a set of questionnaires. The predictability of the COVID-19 severity on RSFA abnormality was tested using a multivariate approach.
Further, the level of RSFA abnormalities was correlated to measures of physical, cognitive, and mental functioning (PCM) using regression. Also, the spatial overlap between COVID-19-related cerebrovascular burden maps and a range of brain metabolic, neurotransmitter, protein expression (including angiotensin-converting enzyme-2 [ACE2]; neuropilin-1 [NRP1]; neuropilin-2 [NRP2]) and cell-type parameters were also assessed.
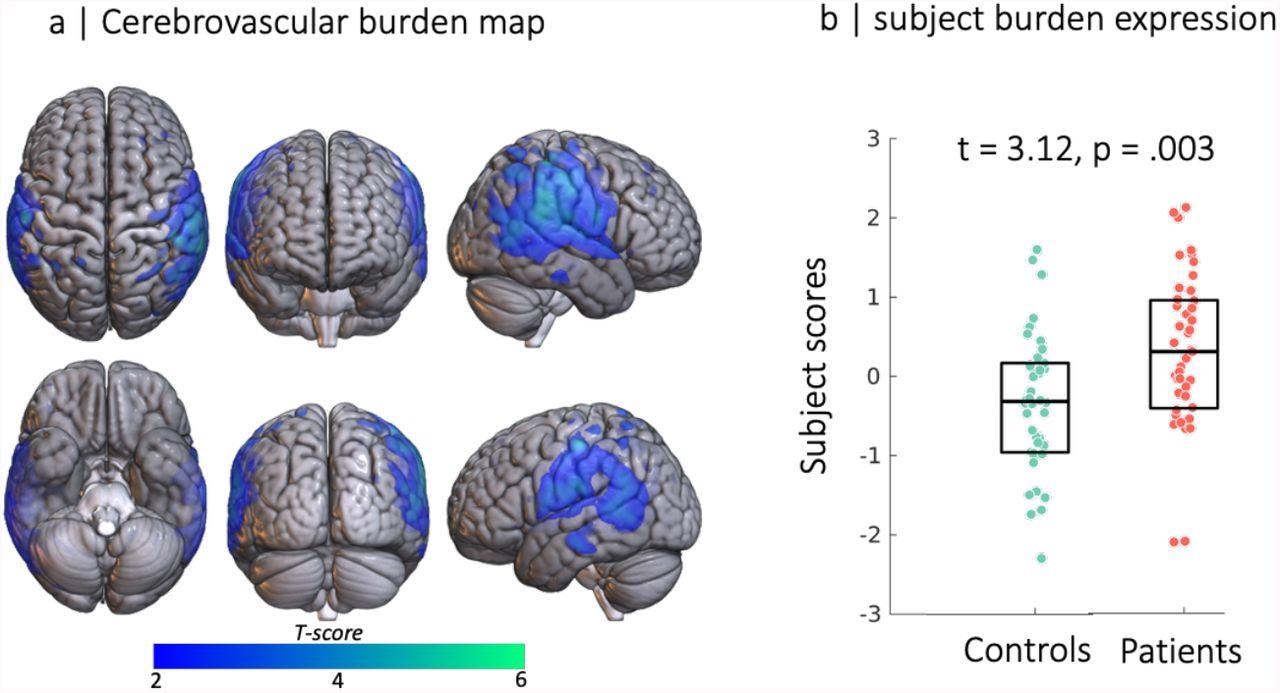
Source-based cerebrovasculometry for the component differentially expressed between groups: (left) independent component spatial map reflecting decrease in RSFA values in temporo-parietal regions. (right) Bar plot of subject scores for patients hospitalised for COVID-19 (red) and control group (green, each circle represents an individual) indicating higher loading values for patients than controls as informed by two-sample unpaired permutation test (a robust regression was used to down-weight the effects of extreme data points)
What did the researchers find?
The team observed that the cerebrovascular abnormality, measured using resting-state fluctuation amplitudes (RSFA), persisted many months after hospitalization for acute COVID-19.
The location of these abnormalities was found to be in lateral, frontal, and temporoparietal regions of the brain, which aligns partially with the cerebrovascular dysfunction reported in association with aging and preclinical Alzheimer’s disease.
These post-COVID-19 effects on cerebral microvasculature were related to the severity of the acute illness and the host responses in the acute stage. In addition, these effects are also associated with the post-COVID-19 cognitive function, mental health, and quality of life six months after hospitalization.
An overlap was observed between the regional distribution of cerebrovascular abnormality, the spatial distribution of the 5-HT1b vasoreactive receptor, and regions with high metabolic demands. Previous reports have implicated loss of 5-HT1b in progressive cognitive loss, which may be pertinent for late consequences of COVID-19. However, it is still unknown whether the localization of RSFA abnormalities to regions rich in 5HT-1b receptors is a consequence of overactivity of these receptors (with consequential low cerebral blood flow), or loss of these receptors (with consequential vasoparalysis or inflammation), or a manifestation of flow-metabolism mismatching with inadequate substrate and oxygen delivery. This is relevant as potential therapeutic agents that are available modulate both 5HT-1b function and inflammatory response.
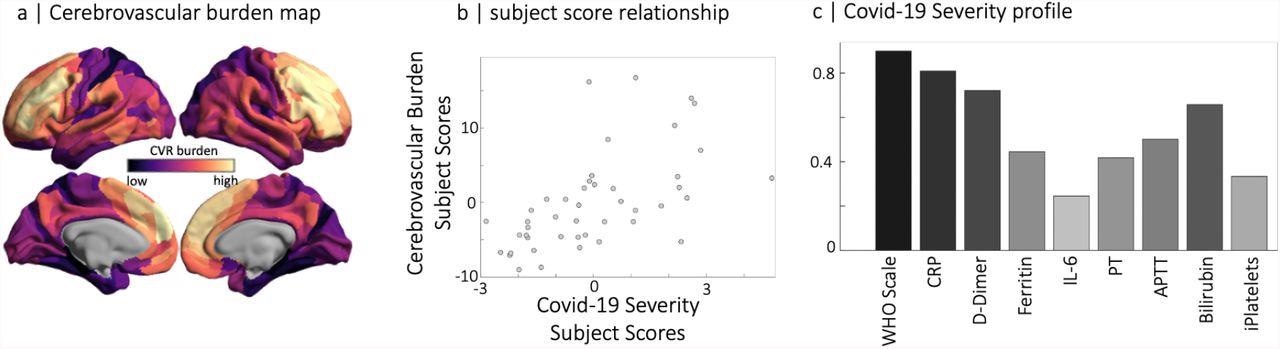
Partial least squares analysis of COVID-19 severity data at the acute stage and RSFA-based cerebrovascular burden (CVB) at the chronic stage. (panel a) Spatial distribution of parcellated RSFA values where dark to light colors are used for the strength of positive and negative correlations with the COVID-19 Severity profile (panel c). Note that regions with a high cerebrovascular burden have low values in RSFA. The scatter plot in the middle panel represents the relationship between subjects' scores of RSFA-latent variable and the COVID-19 Severity-latent variable identified by partial least squares analysis.
Based on the findings, the team correlates cerebrovascular abnormalities with the disease severity via two potential interpretations. One points towards the possibility that alterations in cerebrovascular integrity are the consequences of direct viral invasion, while the other points towards the consequence of the host inflammation in response to viral invasion.
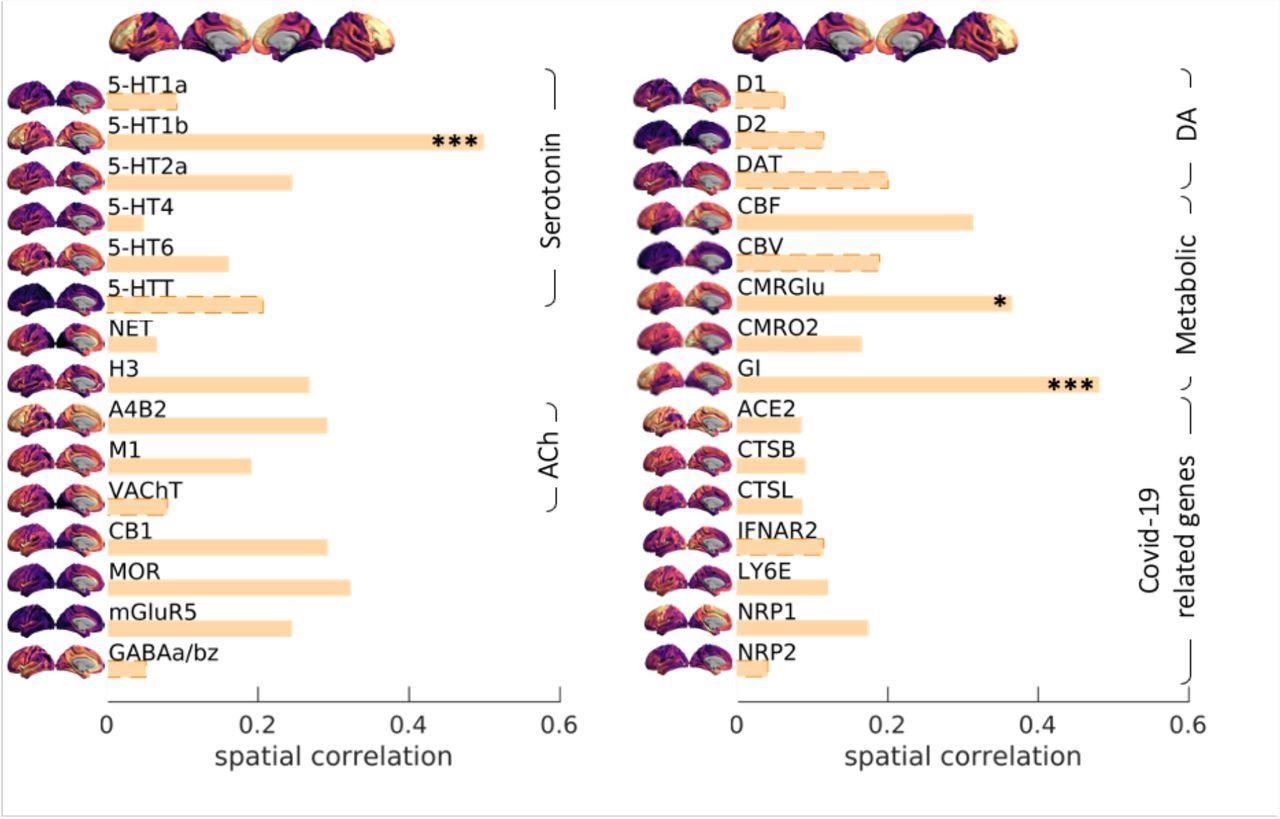
Spatial correlation between Covid19 severity-induced cerebrovascular burden map and spatial patterns associated with a range of neurotransmitter receptor/transporters (Hansen et al., 2021b), selective genes relevant to SARS-CoV-2 brain entry (Iadecola et al., 2020) and brain metabolism parameters (Vaishnavi et al., 2010). Neurotransmitter receptors and transporters were selective to serotonin (5-HT1a, 5-HT1b, 5-HT2a, 5-HT4, 5-HT6, 5-HTT), norepinephrine (NET), histamine (H3), acetylcholine (ACh, A4B2, M1, VAChT), cannabinoid (CB1), opioid (MOR), glutamate (mGluR5), GABA (GABAa/bz) and dopamine (D1, D2, DAT). Metabolic maps were based on cerebral blood flow (CBF), cerebral blood volume (CBV), the cerebral metabolic rate of glucose and oxygen (CMRGlu, CMRO2) and glycemic index (GI). Selective genes relevant to SARS-CoV-2 brain entry included angiotensin-converting enzyme-2, ACE2; neuropilin-1, NRP1; neuropilin-2, NRP2, cathepsin-B, CTSB; cathepsin-L, CTSL, interferon type 2 receptors, IFNAR2; lymphocyte antigen 6-family member E, LY6E. The spatial maps of 5-HT1b, CMRGlu and Glycemic Index (GI) were significantly correlated with Covid19 severity-induced cerebrovascular burden map (* p-spin<0.05 (one-sided), *** p-spin<0.001). See text for more information.
The team did not find elevated expression of genes involved in cellular responses to viral invasion, especially ACE2 and Neuropilin-1. Spatial correlation of RSFA abnormality with the expression of such genes would have provided supportive evidence for the involvement of direct viral infection as a mechanism. But as the team could not demonstrate such a correlation, this adverse finding favors the interpretation that host inflammatory responses could drive the late cerebrovascular dysfunction and merit further investigation.
“The association of microvascular abnormalities with late outcomes of relevance to patients, and the fact that they represent an easily accessible biomarker, suggest both a potential therapeutic target and/or a biomarker of treatment effect in interventional studies”, concludes the team.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Tsvetanov K., A. et al. (2022) Hospitalization for COVID-19 predicts long lasting cerebrovascular impairment: A prospective observational cohort study, medRxiv, doi: https://doi.org/10.1101/2022.02.01.22270235, https://www.medrxiv.org/content/10.1101/2022.02.01.22270235v1
Posted in: Men's Health News | Medical Research News | Medical Condition News | Women's Health News | Disease/Infection News
Tags: ACE2, Aging, Angiotensin, Anosmia, Antigen, Biomarker, Blood, Brain, Cannabinoid, Cell, Chronic, Cognitive Function, Coronavirus, Coronavirus Disease COVID-19, Delirium, Disability, Dopamine, Electronic Medical Records, Encephalitis, Enzyme, Genes, Genetic, Glucose, Glycemic Index, Histamine, Imaging, Inflammation, Interferon, Lymphocyte, Magnetic Resonance Imaging, Mental Health, Metabolism, Neurodegeneration, Neuroimaging, Neurology, Norepinephrine, Oxygen, Pathophysiology, Preclinical, Protein, Protein Expression, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Serotonin, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Stroke, Syndrome, Vascular

Written by
Namita Mitra
After earning a bachelor’s degree in Veterinary Sciences and Animal Health (BVSc) in 2013, Namita went on to pursue a Master of Veterinary Microbiology from GADVASU, India. Her Master’s research on the molecular and histopathological diagnosis of avian oncogenic viruses in poultry brought her two national awards. In 2013, she was conferred a doctoral degree in Animal Biotechnology that concluded with her research findings on expression profiling of apoptosis-associated genes in canine mammary tumors. Right after her graduation, Namita worked as Assistant Professor of Animal Biotechnology and taught the courses of Animal Cell Culture, Animal Genetic Engineering, and Molecular Immunology.
Source: Read Full Article