Assessing risk of vascular inflammation for diabetes patients
A team of international scientists from Nanyang Technological University, Singapore (NTU Singapore), Tan Tock Seng Hospital (TTSH) in Singapore, and the Massachusetts Institute of Technology (MIT) has developed a simple method of extracting tiny biological particles from a person’s blood and use them as biomarkers to assess the health of their blood vessels.
The biomarkers are nanoscale particles called extracellular vesicles (EVs) that are released by specific cells into the bloodstream. Their job is to transport biomaterials, such as proteins and nucleic acids from one cell to another.
In a paper published in June 2021 in Royal Society of Chemistry—Lab on a Chip, the team showed that the blood samples from several diabetes patients had an abnormally high amount of circulating EVs secreted by immune and platelet cells (10 to 50 times more), compared to other diabetes patients.
The team found in lab experiments that when these EVs (taken from the six patients with high EV counts) were added to vascular cells, they induced higher levels of vascular inflammation markers. The results suggest that patients with high EVs in their blood may possibly be at higher risk of developing vascular complications in the long term.
The findings were reported by an interdisciplinary team jointly led by Assistant Professor Hou Han Wei from the School of Mechanical and Aerospace Engineering and NTU President Professor Subra Suresh, in collaboration with NTU Materials Science and Engineering Assistant Professor Dalton Tay, Associate Professor Rinkoo Dalan, Senior Consultant, Endocrinology, TTSH, and MIT Senior Scientist and NTU Visiting Professor, Dr. Ming Dao.
In their research paper, the team explained how their prototype “lab-on-a-chip” can automatically separate EVs from blood samples in an hour—about one-fifth of the usual time needed using conventional centrifugation methods.
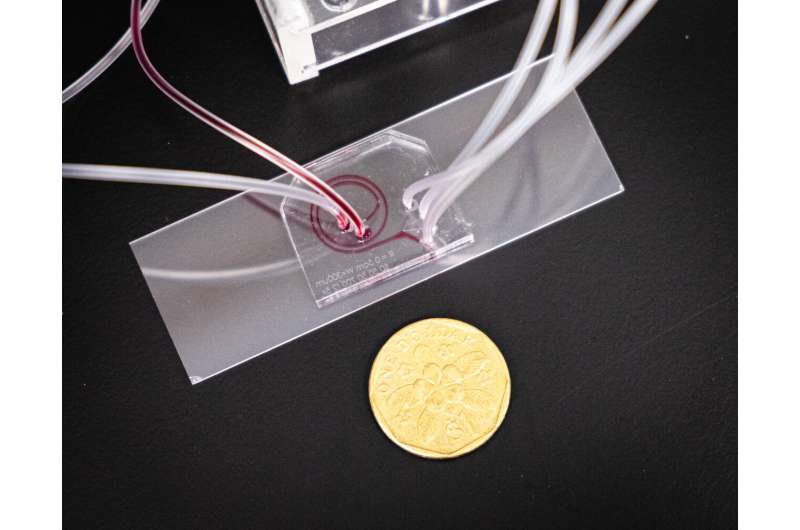
How the new chip works
Named “ExoDFF” (Exosome isolation using Dean Flow Fractionation), the microfluidic chip first sends a blood sample through a spiral-shaped channel at a high speed.
Based on centrifugal and inertial effects—the motion of fluid and the hydrodynamic forces acting on particles in the fluid—bigger blood cells are spun off in one direction while the smaller EVs will flow faster and are directed to a different exit for collection.
The current global benchmark for separating EVs from blood using ultracentrifugation is time-consuming (up to five hours) and captures very few EVs from the blood sample. It is also laborious and non-standardized as different labs have different protocols for extracting and purifying EVs.
In comparison, using the ExoDFF chip allows the process of EV separation and enrichment to be combined into a single step, and does not require trained expertise, said Asst Prof Hou, also a faculty at NTU’s Lee Kong Chian School of Medicine.
“We worked closely with our TTSH clinician partner to ensure that one-step operation of the chip is all that is needed to easily extract the EVs for analysis. Microfluidics is now a mature technology, and with our deep expertise in this field, we can easily design unique microfluidics solutions to isolate various cells and biomarkers from human blood,” says Asst Prof Hou, who previously invented a lab-on-a-chip to analyze a person’s immune system status in 2019.
Potential impact of innovation
Over 422 million people in the world have diabetes and in Singapore, 10 percent of its population (over 400,000) have the metabolic disease, while cardiovascular diseases account for some 31% of the deaths worldwide, including Singapore.
Assoc Prof Rinkoo Dalan, who is also an adjunct professor at the Lee Kong Chian School of Medicine said, “Cardiovascular diseases (CVD) accounted for 18.6 million deaths worldwide in 2019, of which 58% occurred in Asia. Despite significant advances in treatment, the mortality and morbidity associated with atherosclerosis remain high.”
“We need methods to categorize the potential risk for our diabetes and other high-risk patients long before significant damage has happened to the arteries so that we can institute preventive methods. This innovation has the potential to detect the risk early so that preventive methods can then help to limit the progression of damage to the blood vessels.
“This innovation is especially beneficial since it coincides with the development of novel anti-inflammatory agents like canakinumab which has the potential to prevent cardiovascular diseases in these patients, as well as other diabetes-specific medications like SGLT2 inhibitors which can give certain protective effects for the heart. These medications can then be given to patients who are shown to be at a higher risk of developing CVD. The device also has the potential to be used to assess the effect of therapeutics on the arteries.”
Prof Subra Suresh, also the inaugural Distinguished University Professor at NTU, said: “Being able to identify diabetes patients who have increased vascular inflammation could give doctors an edge in treating cardiovascular diseases in its early stages before it evolves into a more serious condition requiring a more invasive treatment involving stents or surgery.”
Currently, the EV separation chip can process up to 5 ml of blood in an hour. As the ExoDFF design is scalable, low-cost (each chip will cost only a few dollars to manufacture), and does not require any chemicals, it can be engineered to process larger sample volumes or adapted for use for manufacturing cell-based or EV-based therapies, such as stem cell therapy.
The team hopes to develop an automated and smaller “printer-sized” version of the machine for clinical and research use.
Source: Read Full Article



