Scientists discover biomarkers and therapies for a group of fatal pediatric neurodegenerative disorders
An exciting study from Texas Children’s Hospital and Baylor College of Medicine has identified molecular biomarkers and two effective approaches to treat Infantile Neuroaxonal Dystrophy (INAD), a devastating and fatal pediatric neurodegenerative disorder.
In a paper published in eLife, distinguished service professor at Baylor College of Medicine, Dr. Hugo J. Bellen, and his research team at the Jan and Dan Duncan Neurological Research Institute (Duncan NRI) at Texas Children’s Hospital used various INAD disease models to discover key molecular signatures of this disorder that will help researchers and clinicians design targeted and effective treatments for patients with INAD.
The study extended these findings further to identify four known drugs and a novel preclinical targeted gene therapy approach that successfully reversed some underlying molecular defects, alleviated disease symptoms, and extended lifespan in INAD animal models.
This work offers an incredible advancement in INAD/PLA2G6-associated neurodegenerative disorders and opens viable and effective therapy options for these currently untreatable disorders.
Disruptions in lipid recycling underlie INAD symptoms
INAD is a rare neurological disorder that causes rapid regression of motor and cognitive abilities in affected children. It is characterized by a loss of muscle tone, seizures, the disintegration of certain brain regions, and visual impairments. Typically, children with INAD do not survive beyond the first decade of their life.
It is caused by variants in the PLA2G6 gene which encodes an enzyme called A2 phospholipase. Variants in PLA2G6 result in INAD and two other later-onset Parkinson-like conditions, atypical NAD (aNAD) and Parkinson’s disease 14 (PARK14), which are collectively referred to as PLA2G6-associated neurodegeneration (PLAN).
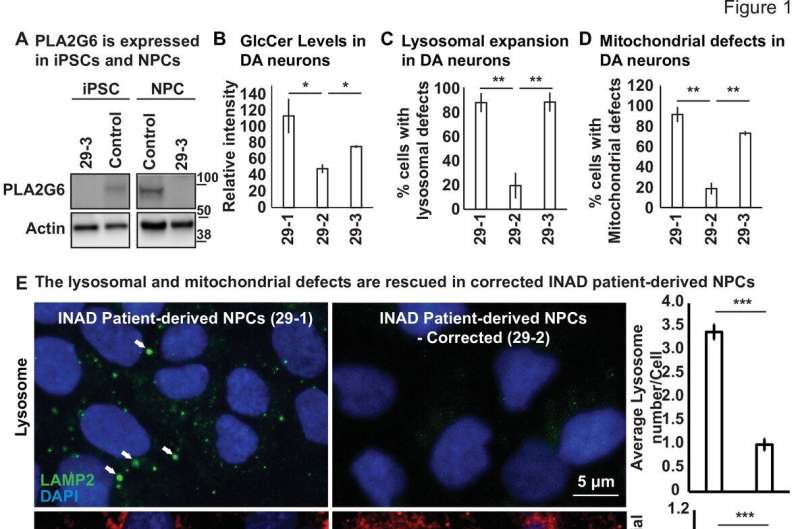
A 2018 study by Dr. Guang Lin and others in the Bellen lab reported that flies lacking the PLA2G6 gene displayed signs of slow progressive neurodegeneration such as shorter lifespan, motor, and vision defects, and presented with cellular characteristics similar to those seen in the neurons of INAD patients. Furthermore, they discovered aberrant recycling and trafficking of cellular lipids as a primary cause of INAD disease symptoms.
To maintain an optimal balance of lipids in the membrane with the least expenditure of energy, cells reuse and recycle membrane lipids such as ceramide phosphoethanolamine (CPE) and sphingomyelin (SM) using intracellular recycling pathways. Retromer proteins are important components of the recycling pathway which extracts various cellular components from endocytosed membranes before they reach the lysosomes, and brings them back to plasma membranes.
CPE and SM that are not recycled are transported to the lysosomes where they are broken down into ceramides, which are then re-used in membranes. The Bellen team found that the absence of PLA2G6 protein in INAD flies destabilized the retromer complex and disrupted its recycling capacity, which caused ceramides to accumulate.
The first question that Dr. Lin et al. tested in this paper was if the INAD cellular defects were evolutionarily conserved across different species.
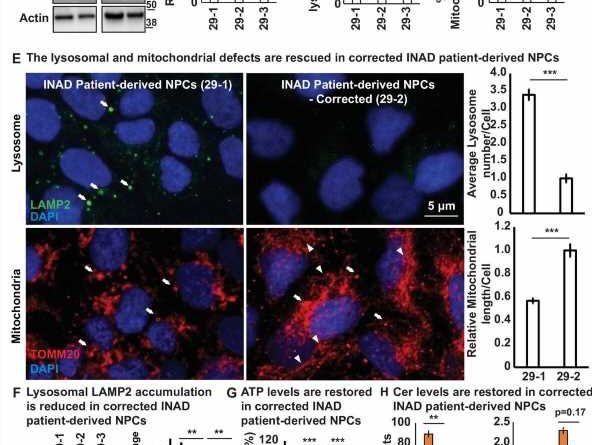
They found that neural progenitor cells derived dopaminergic neurons from patient cells and PLA2G6 mutant mice neurons obtained from the brain regions implicated in INAD exhibited similar cellular defects—accumulation of ceramides, an enlargement of lysosomes along with defects in the structure and function of mitochondria, the energy powerhouse of the cell—indicating their critical role in the pathogenesis of INAD/PARK14.
Four known drugs reverse disease symptoms in INAD flies and patient-derived neuronal cells
Their next goal was to identify therapeutic strategies for INAD. They first reviewed the medical literature to find drugs that had been reported to regulate sphingolipid metabolism, intracellular protein trafficking and treat Parkinson’s disease. Using these criteria, they found twenty potential drug candidates whose efficacy was tested in INAD flies using a bang-sensitivity assay that serves as a readout for seizures.
“Of these, four known drugs showed a dose-dependent improvement in susceptibility to seizures in these flies and did not exhibit any signs of toxicity in INAD patient-derived neural progenitor cells,” said Dr. Lin who is an assistant professor in the Bellen lab.
“Furthermore, these drugs also showed a significant reversal in PLA2G6-induced cellular defects and reduced ceramide levels as well as the size of the lysosomes, indicating a restoration in the flow of intracellular traffic flow”. It is encouraging that three of those drugs are used to treat other disease conditions.
Preclinical gene therapy suppresses motor deficits in INAD patient-derived cells and mouse models
Their next goal was to test if a gene therapy approach would be effective in treating INAD patients.
The Bellen team had previously found that whole-body expression of the human version of the PLA2G6 gene in the INAD fly model reverts the symptoms of neurodegeneration and restores normal lifespan. However, specific expression of this transgene only in neurons rescued the neurodegenerative phenotypes but did not prolong lifespan. This suggested that although this gene clearly plays a critical role in the nervous system, it is also required in other cells.
Based on that observation, they designed an adeno-associated viral (AAV)-based gene therapy approach that would deliver the human PLA2G6 gene to as many cell types as possible. Interestingly, expression of these viral vectors in INAD patient-derived neural progenitor cells induced low-level expression of human PLA2G6 which was sufficient to partially alleviate two key symptoms: lysosomal expansion and mitochondrial structural defects.
Encouraged by these promising results, the team injected human PLA2G6 into the cerebrospinal fluid (of the brain ventricles) and bloodstream and found that the expression of this transgene was not only safe for the mice but that it was also successful in delaying the onset of motor defects and in prolonging the lifespan of adult pre-symptomatic INAD mice.
“We hope this study will not only advance our understanding of the underlying mechanisms involved in INAD/PLA2G6-associated disorders but that these valuable insights will garner more interest in this group of fatal disorders and accelerate therapy development, which is an urgent need for the affected children and their families,” Dr. Bellen said.
More information:
Guang Lin et al, Exploring therapeutic strategies for infantile neuronal axonal dystrophy (INAD/PARK14), eLife (2023). DOI: 10.7554/eLife.82555
Journal information:
eLife
Source: Read Full Article