Study links severe COVID-19 disease to short telomeres
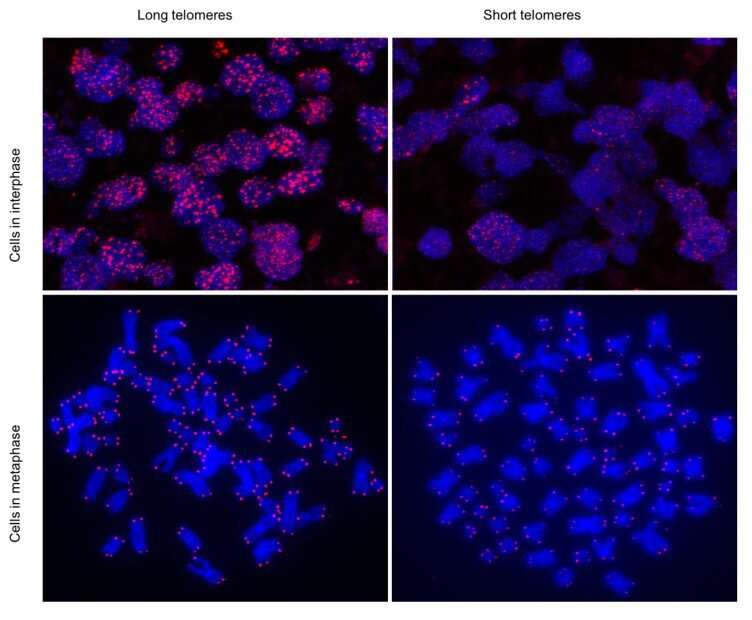
Patients with severe COVID-19 disease have significantly shorter telomeres, according to a study conducted by researchers at the Spanish National Cancer Research Centre (CNIO) in collaboration with the COVID-IFEMA Field Hospital, published in the journal Aging. The study, led by Maria A. Blasco and whose first authors are Raúl Sánchez and Ana Guío-Carrión, postulates that telomere shortening as a consequence of the viral infection impedes tissue regeneration and that this is why a significant number of patients suffer prolonged sequelae.
Blasco was already developing a therapy to regenerate lung tissue in pulmonary fibrosis patients; she now believes that this treatment -which should still take at least a year and a half to become available- could also help those who have lung lesions remaining after overcoming COVID-19.
Telomeres and tissue regeneration
The Telomeres and Telomerase Group, led by Blasco at the CNIO, has been researching the role of telomeres in tissue regeneration for decades. Telomeres are structures that protect the chromosomes within each cell of the organism. It is known that telomere length is an indicator of aging: each time a cell divides, its telomeres shorten until they can no longer perform their protective function and the cell, which now becomes damaged, stops dividing. Throughout life, cells are constantly dividing to regenerate tissues, and when they stop doing so because the telomeres are too short, the body ages.
In recent years, researchers have shown in mice that it is possible to reverse this process by activating the production of telomerase, which is the enzyme in charge of making the telomeres longer. In animals, telomerase activation is effective in treating diseases associated with aging and telomere damage, such as pulmonary fibrosis.
COVID-19 as a regenerative disease
In pulmonary fibrosis the lung tissue develops scars and becomes rigid, causing a progressive loss of breathing capacity. The CNIO group has shown in previous studies that one of the causes of the disease is damage to the telomeres of the cells involved in regenerating the lung tissue, the alveolar type II pneumocytes. And these are precisely the cells that the SARS-CoV-2 coronavirus infects in lung tissue.
“When I read that type II alveolar pneumocytes were involved in COVID-19, I immediately thought that telomeres might be involved,” says Blasco.
In the Aging paper, the researchers write: “It caught our attention that a common outcome of SARS-CoV-2 infection seems to be the induction of a fibrosis-like phenotype in lung and kidney, suggesting that the viral infection may be exhausting the regenerative potential of tissues.”
The authors propose that it is the short telomeres that hamper tissue regeneration after infection. As Blasco explains, “we know that the virus infects alveolar type II pneumocytes and that these cells are involved in lung regeneration; we also know that if they have telomeric damage they cannot regenerate, which induces fibrosis. This is what is seen in patients with lung lesions after COVID-19: we think they develop pulmonary fibrosis because they have shorter telomeres, which limits the regenerative capacity of their lungs.”
Samples of patients in a field hospital
The data presented in the ‘Aging’ paper provide evidence in favor of this hypothesis, by finding an association between greater severity of COVID-19 and shorter telomeres.
Despite the difficulties arising from conducting research at the height of the pandemic -“the hospital facilities for COVID-19 patients were overwhelmed,” Blasco says- it was possible to analyze the telomeres of 89 patients admitted to the field hospital at the IFEMA in Madrid using several techniques.
As in the general population, the average length of the telomeres decreased as age increased in the patients studied. Furthermore, as the most severe patients are also the oldest patients, there is also a correlation between greater severity and shorter telomere length.
What could not be foreseen, and this is the most important finding, is that the telomeres of the most seriously ill patients were also shorter, irrespective of age.
The researchers write: “Interestingly, we also found that those patients who have more severe COVID-19 pathologies have shorter telomeres at different ages compared to patients with milder disease.”
And they add: “These findings demonstrate that molecular hallmarks of aging, such as the presence of short telomeres, can influence the severity of COVID-19 pathologies.”
Gene therapy for patients with post-COVID-19 pulmonary injury
The intention of the researchers is now to demonstrate a causal relationship between reduced telomere length and pulmonary sequelae of COVID-19. To do this, they will infect mice that have short telomeres and are not able to produce telomerase with SARS-CoV-2; without telomerase, the telomeres cannot be repaired and as a consequence lung tissue regeneration cannot take place. If the hypothesis of Blasco’s group is correct, mice with short telomeres and without telomerase should develop more severe pulmonary fibrosis than normal mice.
Confirmation that short telomeres hamper the recovery of severe patients would open the door to new treatment strategies, such as therapies based on telomerase activation.
“Given that short telomeres can be made longer again by telomerase, and given that in previous studies we have shown that telomerase activation has a therapeutic effect on diseases related to short telomeres, such as pulmonary fibrosis, it is tempting to speculate that this therapy could improve some of the pathologies that remain in COVID-19 patients once the viral infection has been overcome, such as pulmonary fibrosis.”
Source: Read Full Article



