Researchers show how cancer cells resist chemotherapy
A study in human cell lines reveals cancer cells can activate a force-generating mechanism to survive a cancer therapy.
Cancer is a complicated disease—its cells have many ways to evade cancer treatment. Now, new research reveals for the first time how cancer cells circumvent a common cancer therapy—and how targeted drugs could be developed to help combat it.
The study, published in the journal Current Biology, shows cancer cells can activate a force-generating rescue mechanism to stabilize an essential cell structure responsible for cell division and resist the effects of chemotherapy.
The research team, which included experts from UNSW Sydney and University of Technology Sydney (UTS), now hope to use the findings to improve cancer therapies—and they have established a company to develop drugs that specifically target the mechanism.
“We discovered that cancer cells use the mechanical force supplied by the edge of the cell called the cell cortex, to overcome the impact of commonly used chemotherapy that blocks the ability of the cell to separate the chromosomes during cell division,” says Professor Peter Gunning, senior author of the study from the School of Biomedical Sciences, UNSW Medicine & Health.
“Now that we understand this exact pathway cancer cells use to avoid the toxic effects of the chemotherapy, it opens the door to improving cancer treatments.”
Unpacking the mechanism
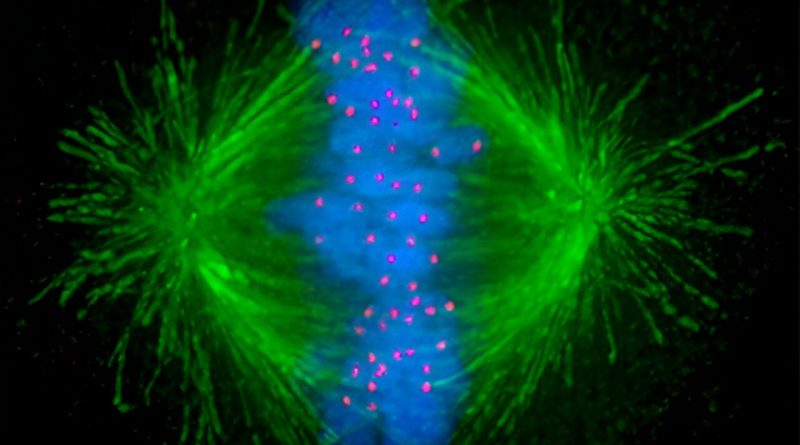
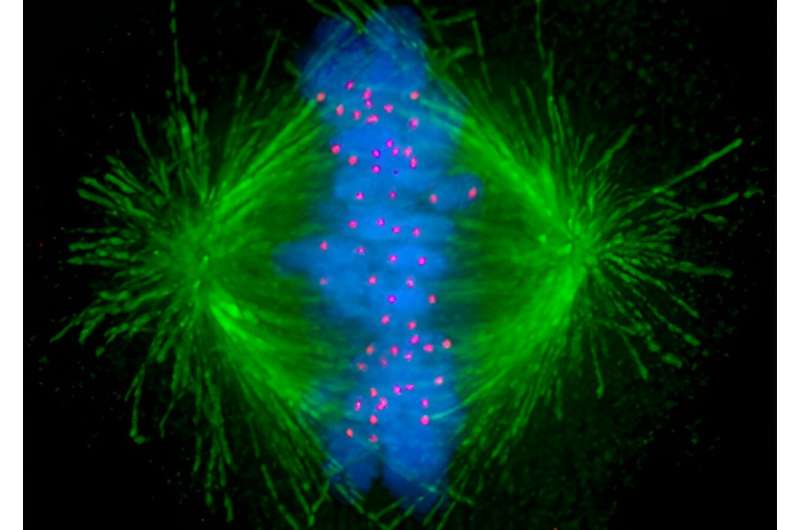
During cell division, or mitosis, microtubules—tiny tube-like structures inside the cell—act as arms for separating genetic material evenly and ensuring the successful production of daughter cells. Cancer cells rapidly divide—much more so than normal cells—so targeting these structures using anti-microtubule chemotherapy drugs is commonly used to inhibit their growth.
“The anti-microtubule chemotherapy usually fragments the mechanical arms into multiple hubs that pull the chromosomes in multiple directions rather than the normal two. The resulting chaos prevents proper separation of the chromosomes to the two daughter cells and induces apoptosis, or programmed cell death,” Prof. Gunning says.
“At high doses of chemotherapy this is quite effective. But at lower exposure, which is a core problem, particularly as you get to the interior of solid tumors or when chemotherapy doses must be reduced due to toxicity in patients, cancer cells activate a rescue mechanism.”
Prof. Gunning says we now know that this mechanism arises because the cancer cells activate a signal that recognizes microtubule fragmentation and causes the arms to reach toward the edge of the cell and pull on the cortex to bring the fragments back together.
“This allows the arms to stabilize and generate the force necessary to physically grab and pull the chromosomes into each daughter cell and ensure the cancer cell multiplies.”
The researchers initially suspected the mechanism existed after noticing a specific drug combination that targeted the microtubules enhanced chemotherapy for a childhood cancer called neuroblastoma. But to understand exactly how it worked, the researchers needed to use advanced imaging to observe the mechanism in action.
“We needed good imaging of the cancer cells as they go through cell division to visualize what’s happening to the chromosomes, the microtubules and the architecture of the cell in real-time,” Prof. Gunning says. “It was quite surprising to us because we did not expect this mechanism of the cancer cell to be used in this way to overcome the cancer therapy, but we could see it happening before our eyes.”
Prof. Gunning says the mechanism is likely to be a fundamental component of cell biology.
“We think it’s a fallback mechanism that evolved to allow any cell to overcome a small amount of microtubule disruption and ensure it can survive,” Prof. Gunning says. “It just so happens cancer cells use it to sidestep the anti-microtubule chemotherapy.”
The researchers will now focus on developing drugs that work in combination with current chemotherapy agents to overcome the cancer cell resistance mechanism. But first, they need to improve the activity of the drugs in animal models in the next few years before advancing into preclinical studies and eventually into patients.
“By attacking the force-generating machinery built by the cancer cells we expect that we will be able to allow the cancer therapy to do its job much more effectively,” Prof. Gunning says. “In practical terms, we have established a company that will allow us to develop the drugs needed to attack this rescue mechanism, enabling the anti-microtubule chemotherapy to act more effectively, and hopefully improve patient outcomes.”
More information:
Dejiang Wang et al, Cortical tension drug screen links mitotic spindle integrity to Rho pathway, Current Biology (2023). DOI: 10.1016/j.cub.2023.09.022
Journal information:
Current Biology
Source: Read Full Article