Malaria-causing parasites resistant to both treatment and detection have emerged in Ethiopia
Scientists have detected new strains of malaria-causing parasites in Ethiopia that are both resistant to current treatments and escape detection by common diagnostic tests—a development that could increase cases and deaths from malaria and make eliminating the persistent disease an even greater challenge.
The authors detailed their findings from a genomic surveillance study in Nature Microbiology. Already, scientists had found in Uganda, Tanzania and Rwanda strains of the parasite that causes malaria that were resistant to most available antimalarial drugs; and separately, malaria parasites resistant to diagnostic tests had emerged in the Horn of Africa.
Those parasites have been spreading independently of one another, but the new study is the first published report to confirm the prevalence of this type of double-resistant malaria strain, said study author Jeffrey Bailey, an associate professor of translational research and pathology and laboratory medicine at Brown University.
“Now we’re essentially seeing the worst-case scenario: parasites with the mutation that make them resistant to treatment have also picked up the chromosomal deletions that make them invisible to the diagnostic tests,” Bailey said. “This means that it will be harder to detect people who are infected, and then when infected people are treated with antimalarial drugs, that may not work to stop them from spreading the disease.”
The standard method to diagnose malaria in Africa is through rapid diagnostic tests that detect specific parasite proteins in the blood that are highly expressed. The tests can confirm malaria even if the patient is asymptomatic. The parasites lacking the genes for these proteins have evolved to be invisible to the tests.
The first-line malaria treatment recommended by the World Health Organization is a combination therapy involving artemisinin-based drug compounds, which tend to be very effective in preventing death and reducing transmission. The mutations now detected in Africa provide resistance to artemisinin.
Bailey’s research team at Brown, in close collaboration with the researchers from the Ethiopian Public Health Institute and the University of North Carolina at Chapel Hill, conducted a comparative genomic analysis of malaria-parasite samples with the deleted protein-expressing genes that had been collected across three regions of Ethiopia.
Led by Bailey, co-director of the Ph.D. program at Brown’s Center for Computational Molecular Biology, the scientists used molecular sequencing to assess the prevalence of mutations that confer resistance to artemisinin. Abebe Fola, a postdoctoral researcher in Bailey’s lab, was instrumental in this work and is the first author of the paper.
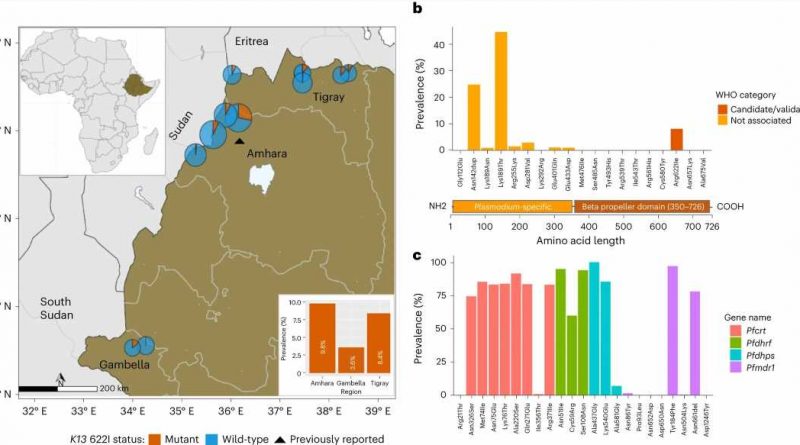
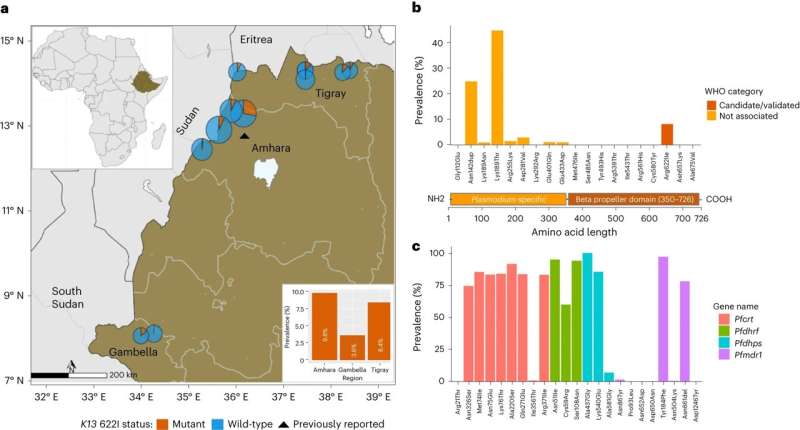
They found that 8.2% of drug-resistant parasites also carried the deletions of the protein-expressing gene that made them detectable by the diagnostic tests.
In Ethiopia, the overall incidence of malaria is low, but the disease remains endemic in 75% of the country, with 65% of the population at risk. More than 5 million episodes of malaria occur each year. The Ethiopian government set a goal for malaria elimination by 2030, and prompt diagnosis and treatment with effective drugs is a cornerstone of the malaria elimination program.
“The spread of these parasites will certainly make eliminating malaria in Ethiopia and elsewhere in Africa more difficult and will likely lead to increased cases and deaths,” Bailey said.
The scientists concluded that close monitoring of the spread of combined drug- and diagnostic-resistant parasites is needed, noting that improved understanding of how these mutations emerge, interact and spread is critical to the success of future malaria control and elimination efforts across Africa.
In addition, Bailey said, there is an urgent need to develop new therapies, in addition to artemisinin, to treat malaria as well as vaccines to prevent and slow the spread of the disease.
The ability to conduct genomic surveillance to monitor mutations while looking for new ones has greatly advanced over the last decade, Bailey said, with the emergence and refinement of next-generation sequencing. His lab at Brown has pioneered high-throughput techniques to sequence many genes at once, and has been collaborating on projects like the current study with research teams at other universities as well as health agencies in countries such as Uganda.
While the analysis for this study was conducted at Brown, Bailey and others on the research team are working on building the capacity for genomic surveillance in Ethiopia and other parts of Africa.
More information:
Abebe A. Fola et al, Plasmodium falciparum resistant to artemisinin and diagnostics have emerged in Ethiopia, Nature Microbiology (2023). DOI: 10.1038/s41564-023-01461-4
Journal information:
Nature Microbiology
Source: Read Full Article