Lethal cancer cells buddy up to survive
Tumor cells in the most common pancreatic cancer share nutrients to live and grow. A new discovery by University of California, Irvine biologists and collaborators during a four-year investigation could help lead to better treatments for pancreatic ductal adenocarcinoma, which accounts for over 90 percent of pancreatic cancer cases. The scientists’ paper appears in Nature Cancer. While pancreatic cancer is relatively rare, it is among the leading causes of cancer death in the United States.
One obstacle in treating pancreatic ductal adenocarcinoma, known as PDA, is that it generally does not show early symptoms. Another hurdle is the complexity of its dense and fibrous tumors. Consequently, they do not have fully functioning blood vessels in the tumor. On one front, this makes it difficult to deliver effective chemotherapy. However, it also means the tumors have developed a different kind of metabolism.
“Without blood vessels, PDA cells aren’t getting the normal nutrients they need, so they have come up with other ways to nourish themselves and grow,” said Christopher Halbrook, assistant professor of molecular biology & biochemistry, and lead and co-corresponding author. Understanding this process is essential for devising treatments targeting the cancer’s metabolism.
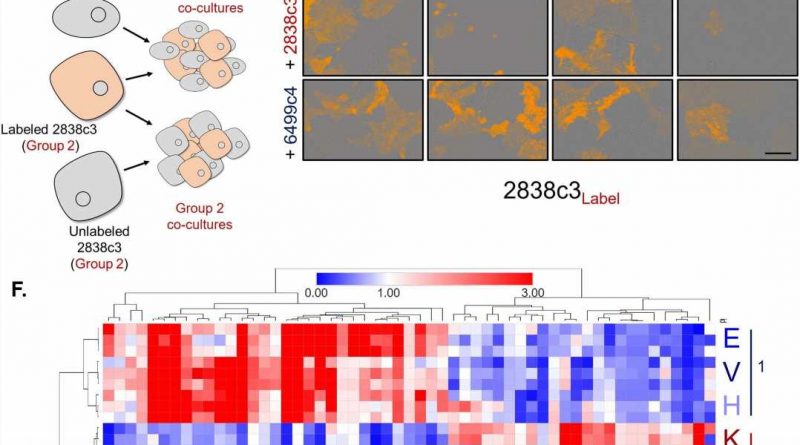
The researchers discovered two different PDA types of cancer cells exist within tumors, each with distinct metabolic processes. “This differential programming allows them to exchange nutrients, so each gets what it needs,” Halbrook said.
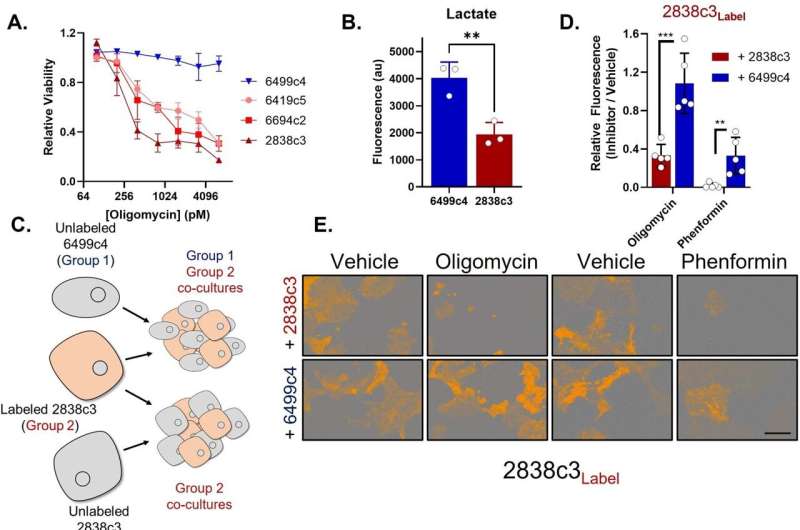
The team found that one class of cells is susceptible to mitochondrial poisons, often used as cancer treatment, yet it survives with the help of the amino acid asparagine it receives from the other cell class. The latter have plenty to share because they overproduce asparagine in response to ongoing stress.
“After learning this, we discovered we could use the enzyme asparaginase to reestablish the tumors’ vulnerability to mitochondrial poisons,” said Halbrook.
The team’s findings yield important knowledge for improving treatment of pancreatic cancer and potentially for other diseases. “With tumors, we can’t assume there is a homogeneous weakness to target,” said Halbrook. “If we are going to have metabolism-based therapies, it will be crucial to aim at multiple cell behaviors.”
The scientists validated the metabolic symbiosis model using cloned human and mouse PDA tumor cells and single-cell RNA sequencing. Then using pre-clinical models, they made another important finding.
“When we treated tumors with Phenformin, a potent mitochondrial poison, together with asparaginase, it stopped tumor growth,” said Halbrook. He added there is more to learn, including identifying the cause of the ongoing stress in the support cells driving the symbiosis.
More information:
Christopher J. Halbrook et al, Differential integrated stress response and asparagine production drive symbiosis and therapy resistance of pancreatic adenocarcinoma cells, Nature Cancer (2022). DOI: 10.1038/s43018-022-00463-1
Journal information:
Nature Cancer
Source: Read Full Article