From soft tissue to stiff leather: Understanding the role of paxillin in liver fibrosis
Currently, the United States lacks FDA-approved treatments for liver fibrosis, highlighting the critical need to understand the cellular biology and pathways associated with this condition.
In a recent study led by Don Rockey, M.D., the director of the Digestive Disease Research Core Center, and Nour Hijazi, an M.D.-Ph.D. student at the Medical University of South Carolina, significant progress has been made in understanding a pathway contributing to liver fibrosis. Their findings, highlighting a potential novel therapeutic pathway for liver fibrosis, were recently published in the Journal of Cell Science.
Liver injury occurs after various insults to the liver, such as alcohol exposure, viral hepatitis, and metabolic abnormalities leading to a fatty liver. The liver in a healthy state is dynamic and flexible, employing strategic mechanisms to initiate healing when injuries occur. However, persistent damage can trigger the gradual development of scar tissue known as fibrosis.
“Advanced fibrosis of the liver is comparable to stiff, inflexible leather, restricting the capacity of the liver to carry out its essential functions,” explains Rockey.
Liver fibrosis is a global concern, claiming two million lives annually and is currently ranked as the 9th-leading cause of death by the Centers for Disease Control and Prevention. Hijazi informs us of a striking projection: “By 2050, liver fibrosis is expected to triple from what has been reported in the past few decades, and there has been a significant increase in mortality among the younger population.”
One strategy the liver employs to repair injury and acute damage involves the activation of resident liver cells; however, repetitive liver damage hinders this process. Among the many participants in this injury process are hepatic stellate cells (HSCs), unique in their exclusive activation in response to liver injury.
“In the injured liver, activated HSCs go haywire and synthesize large amounts of extracellular matrix, or scar tissue,” explains Rockey.
The extracellular matrix functions like a framework that holds everything together. Made up of proteins and other cellular components that surround liver cells, it provides support and shapes the liver. Controlled production of the extracellular matrix is beneficial and is essential, but production of excessive amounts leads to liver fibrosis and ultimately cirrhosis.
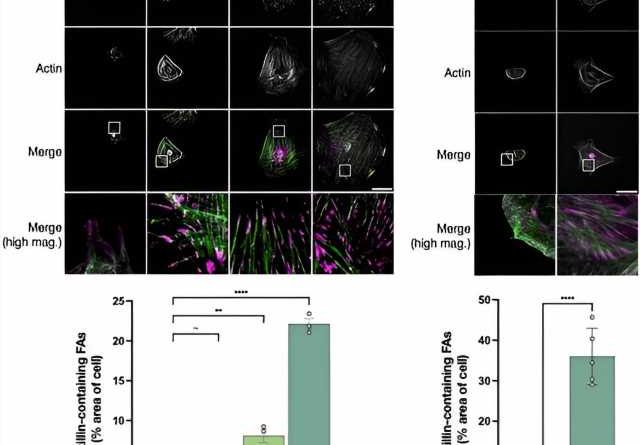
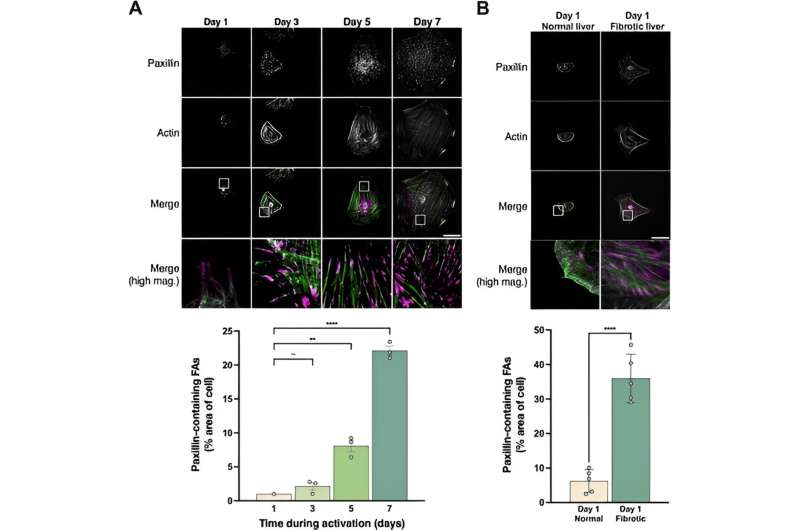
Motivated by the ongoing challenges for liver fibrosis therapies, researchers like Rockey and Hijazi have been investigating pathways that lead to liver fibrosis. Excitingly, the Rockey group has discovered a new role for paxillin, a protein involved in HSC activation.
“Paxillin is a key player in the activation of HSCs and may be setting off a self-continuous loop that signals the machinery responsible extracellular matrix production,” Rockey says.
Identifying a protein in a cycle driving extracellular matrix production holds promise for liver fibrosis therapies. This discovery is especially thrilling considering that liver fibrosis often serves as the common endpoint for various liver diseases, echoing Hijazi’s analogy: “Just like all roads lead to Rome, all liver diseases can lead to fibrosis.”
This new role of paxillin is exciting, and the lab is exploring ways to manipulate paxillin to modify the wound healing response.
“If we can understand the ways that fibrosis develops, then we can contribute to the treatment of many different liver diseases,” emphasizes Hijazi.
More information:
Nour Hijazi et al, Paxillin regulates liver fibrosis via actin polymerization and ERK activation in hepatic stellate cells, Journal of Cell Science (2023). DOI: 10.1242/jcs.261122
Journal information:
Journal of Cell Science
Source: Read Full Article