Flavor your food with ‘flavanols’ to burn excess fat, new study suggests

In cold conditions, brown adipose tissue (BAT) or brown fat generates heat to keep the body warm. Compared with white adipose tissue, BAT has more mitochondria—subcellular organelles associated with energy production—which allows it to burn calories and produce heat by activating the mitochondrial uncoupling protein 1 (Ucp-1). The stimulation of the sympathetic nervous system (SNS) after cold exposure, exercise, and calorie restriction is well known to induce fat browning. Dietary polyphenols may also activate BAT, causing heat to be dissipated from our bodies. BAT activation and white fat browning are thus both therapeutically significant in the fight against cardiovascular diseases and their comorbidities.
A group of scientists examined the browning of fat induced by dietary administration of flavan 3-ols (FLs), a family of “catechin” containing polyphenols abundant in cocoa, apple, grapeseed, and red wine. In a new study published in the journal Nutrients, the team led by Professor Naomi Osakabe of Graduate School of Engineering and Science, Shibaura Institute of Technology, Japan proved that FLs enhance browning of adipose tissue by activating the SNS. The findings revealed a direct correlation between fat browning and FLs consumption, which could help researchers develop new treatments for obesity-related diseases.
The authors of this study had previously discovered that a single oral dose of FLs caused fat burning and increased skeletal muscle blood flow. Here, they investigated the effects of single and multiple dose administration of FLs in mouse adipose tissue and found that FLs activate fat browning via the SNS, which secretes “catecholamine” neurotransmitters such as adrenaline (AD) and noradrenaline (NA). They fed cocoa-derived FLs to distinct groups of mice in two independent sets of experiments. One group was given a single dose of FLs over the course of 24 hours, and their urine was collected for testing. The other group received repeated doses for 14 days before being dissected for the collection of brown and white fat. All adipose samples were tested for gene and protein markers that indicate fat browning, while the urine samples were tested specifically for AD and NA levels.
Higher concentrations of AD and NA in the urine following a single dose of FL clearly demonstrated SNS activation. Although the use of urine samples to evaluate SNS activation is still controversial in clinical research, it has been validated in stressed rodents. “Oral administration of FLs likely activate the SNS because they are considered stressors in these models,” explains Prof Osakabe.
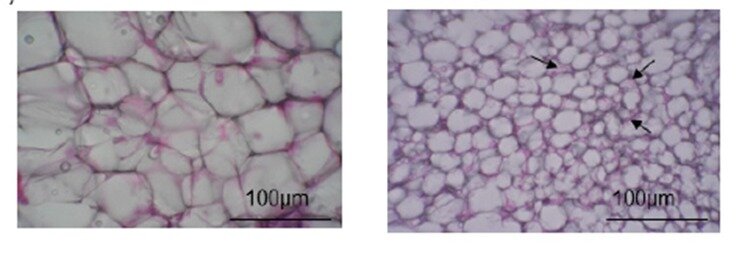
The team then used the obtained adipose tissue to investigate the effects of long-term FL treatment. They were thrilled to discover that the white fat of mice who were fed FLs for 14 days eventually turned brown. Some of these cells also had notable structural changes, such as “multilocular phenotype,” and appeared to be smaller than normal cells. Since BAT dissipates heat energy, does long-term FL consumption change the amounts of heat-related proteins? To answer this question, the scientists showed that Ucp-1 levels, as well as other high temperature-linked proteins, increased in mice fed repeated doses of FLs. Browning markers, referred to as “beige markers” in this study, were also abundant in these mice. “All of these proteins work together to induce the development of the BAT phenotype,” exclaims Prof. Osakabe.
The team believes that the results of their study may contribute to the prevention of lifestyle-related diseases. Interestingly, this is not the first time FLs have worked wonders. Improvements in glucose and insulin tolerance have been seen after just one dose of FL-rich food administration. These findings taken together highlight the need of discussing both the acute and chronic aspects of the metabolic responses generated by FLs consumption.
Source: Read Full Article