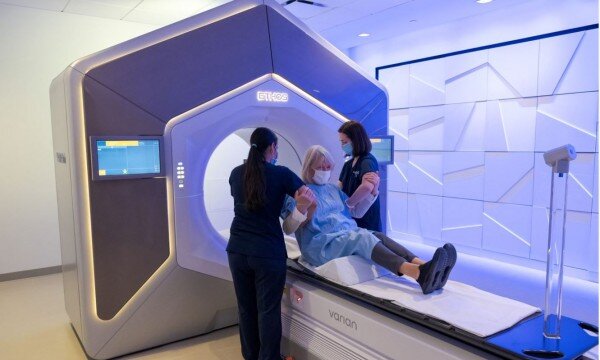
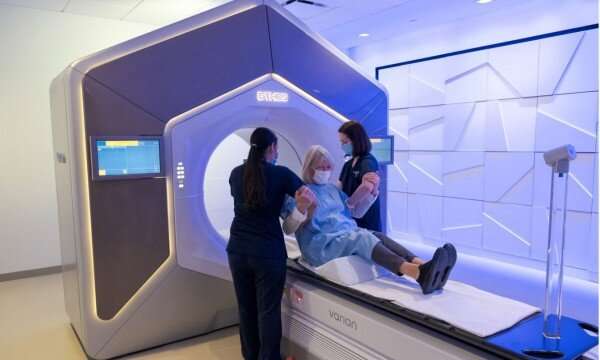
Clinical trial sets stage for new paradigm in kidney cancer treatment
Kidney cancer encompasses a wide spectrum and can present with extensive metastases or just a handful. However, today all patients are treated the same. They all receive medication.
Building upon pioneering work at UT Southwestern Medical Center, investigators report the results of a clinical trial exploring the role of stereotactic ablative radiation therapy (SAbR) for patients with a handful of metastases, or so-called oligometastatic disease. The study represents the first clinical trial for patients with untreated oligometastatic kidney cancer.
“There has never been a clinical trial for these patients. It is unclear whether these patients should be treated with medication, surgery, or another approach. This represents an unmet medical need,” said Raquibul Hannan, M.D., Ph.D., Associate Professor of Radiation Oncology, Immunology, and Urology; Chief of the Genitourinary Radiation Oncology Service; and lead author of the study along with Robert Timmerman, M.D., Professor and Chair of Radiation Oncology, and James Brugarolas, M.D., Ph.D., Professor of Internal Medicine/Hematology-Oncology and Director of the Kidney Cancer Program.
Each year brings more than 430,000 new cases of kidney cancer worldwide and nearly 180,000 deaths, according to the World Health Organization. About 40% of patients develop metastatic disease. Metastatic kidney cancer is typically treated with immunotherapy agents or targeted drugs, which are toxic and diminish quality of life. For most patients, the disease ultimately progresses, necessitating a change in treatment, until patients exhaust their options. When metastatic, most patients eventually succumb to the disease, and the quality of their remaining time is undermined by the cancer drugs.
The phase 2 clinical trial tested SAbR, a treatment that delivers potent, narrow beams of radiation to tumors, in 23 kidney cancer patients with oligometastases (up to five metastases) at UT Southwestern and the affiliated county hospital, Parkland Health. Patients received SAbR to metastatic tumors at the outset and while the disease remained oligometastatic. Overall, 57 metastases were treated. The primary goal was to control the metastatic cancer in at least 60% of patients at one year. The study was successful with more than 90% of patients with disease control at one year without systemic therapy.
“It appears that most patients will be free of systemic therapy for at least two years,” said Dr. Hannan.
None of the patients experienced serious side effects, and periodic questionnaires showed no negative impact on quality of life.
A large phase 3 randomized clinical trial evaluating SAbR for oligometastatic kidney cancer has been approved by the National Cancer Institute and will be led by Dr. Hannan.
“If successful, this phase 3 trial will establish, for the first time, a standard of care for patients with oligometastatic kidney cancer,” said Dr. Brugarolas. “The hope is that the treatment will help patients by delaying progression while preserving their quality of life.”
Source: Read Full Article