Bourneville’s tuberous sclerosis: Everything unfolds in the brain shortly after birth
A Canadian research team has uncovered a new mechanism involved in Bourneville tuberous sclerosis (BTS), a genetic disease of childhood. The team hypothesizes that a mutation in the TSC1 gene causes neurodevelopmental disorders that develop in conjunction with the disease.
Seen in one in 6,000 children, tuberous sclerosis causes benign tumors or lesions that can affect various organs such as the brain, kidneys, eyes, heart and skin. While some patients lead healthy lives, others have significant comorbidities, such as epilepsy, autism and learning disabilities.
Although the role that the TSC1 gene plays in the disease is already known, Montreal scientists have only now identified a critical period in the postnatal development of GABAergic interneurons that are so important to the development of the brain.
The results of their study are reported today in Nature Communications.
An essential ‘pathway’
All mammalian cells, and the proteins that form them, need a ‘pathway’ to regulate their individual growth, which scientists call a ‘signaling pathway,’ explained Clara A. Amegandjin, a doctorate’s student in neurosciences at Université de Montréal and first author of the new study.
“The signaling pathway of mTOR (mechanistic target of rapamycin) controls several aspects of the development of brain cells—the neurons—by regulating different metabolic processes: the proliferation, growth and mobility of neurons, as well as the biosynthesis and transcription of their proteins,” she said.
“The pathway is therefore pivotal in ensuring the development of neurons in an ideal environment.”
When the mTOR signaling pathway is disrupted, certain diseases such as type-2 diabetes, obesity, neurodegeneration and cancer can occur.
“A mutation in the negative regulator of the TSC1 gene of the mTOR pathway is known to produce hyperactivation of the signaling pathway, resulting in abnormal cell proliferation,” said UdeM neurosciences professor Graziella Di Cristo, a researcher at CHU Sainte-Justine children’s hospital.
“This disruption is responsible for neurodevelopmental disorders associated with autism, intellectual disability and epilepsy in tuberous sclerosis, but the underlying mechanisms were not well understood,” said Di Cristo, who oversaw the study.
A conductor that can’t keep time
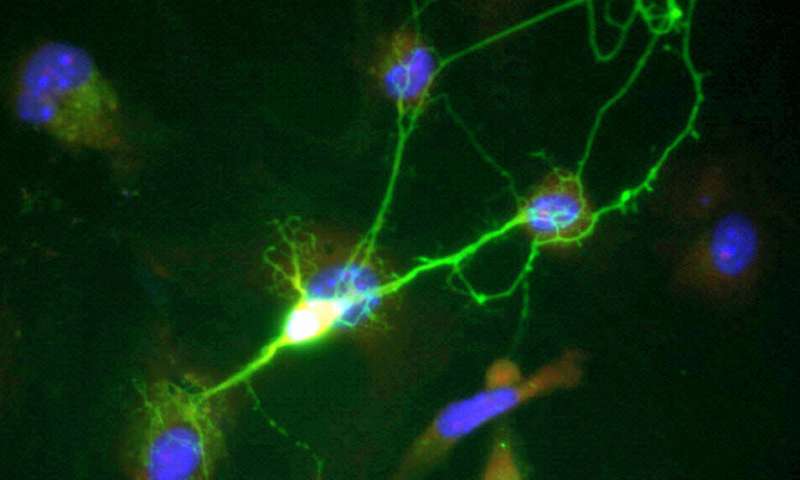
Di Cristo’s laboratory specializes in the study of GABAergic interneurons. This type of neuron acts as a conductor in the cortex by controlling the dynamics of neural networks and circuits that regulate brain function. They are of critical importance for brain development.
“Our original hypothesis was to see if this mutation in the mTOR pathway affected the development of GABAergic cells,” said Amegandjin. “In many cases of autism, these cells are deregulated. However, in tuberous sclerosis, few studies have examined their involvement in the expression of neurological comorbidities.”
Using an organotypic culture that mimics brain development (growth, maturation, and stabilization) ex vivo, the research team introduced the TSC1 gene mutation into GABAergic cells of mice at specific periods during their brain development.
Using biomarkers, the researchers found early and very rapid proliferation occuring in the growth phase of the mutated cells. Synaptic connections that form too quickly become ‘defective’ once they mature.
“We therefore have evidence that neurodevelopmental disorders are mediated by hyperactivity of the mTOR pathway caused by the absence of the TSC1 gene,” said Amegandjin.
Application in humans
Rapamycin is a drug whose mechanism of action is related to the inhibition of the mTOR protein.
“By administering this protein in preclinical models—in this case, mice—we are able to ‘rescue’ synaptic connections and prevent neurodevelopmental disorders,” said Di Cristo. “Based on our results, this therapeutic approach would be most appropriate to prevent premature maturation of neurons.”
Source: Read Full Article



