Bone tissue engineering: Divalent metal cations stimulate new bone formation
The concept of new bone formation via divalent cations is widely reported although the underlying mechanism of the process remains unclear. In a new report now published in Nature Communications, Wei Qiao and a team of scientists in orthopedics surgery, materials science, and biomaterials in China and the U.S. reported how cations can stimulate skeleton interoception to regulate bone formation by promoting prostaglandin E2 secretion (a potent inflammatory mediator) from macrophages. The immune response accompanied sprouting and arborization of nerve fibers to sense inflammatory cues and convey signals to the central nervous system. The process of activating skeleton interoception downregulated the sympathetic tone to facilitate new bone formation. The study revealed how divalent cations promote bone formation via the skeleton interoceptive circuit. This finding can prompt the development of new biomaterials during bone tissue engineering to promote the therapeutic power of divalent cations.
The role of the central nervous system in bone growth and remodeling
In this work, the team hypothesized that communications between the immune system and neural system can trigger skeleton interoception to regulate new bone formation and then sought to characterize such mechanisms of divalent cation-induced bone formation. Various divalent metal cations including magnesium, zinc and copper ions play an important role in bone growth, bone modeling and remodeling. In the past decade, researchers have revealed the regulatory effects of these divalent cations on osteogenesis, osteoclastogenesis, angiogenesis and immune responses. However, recent research has highlighted involvement of the central nervous system during bone formation via divalent cations. Details remain unclear of the central nervous system’s (CNS) involvement, which plays a significant role in bone homeostasis. The CNS (central nervous system) reacts to external stimuli including temperature, sound, odor and taste, while receiving signals from many physiological systems within the body, including the cardiovascular, gastrointestinal and respiratory systems. Recent years have also seen the emergence of interoceptive processes that have regulated the senses to interpret, integrate and regulate signals from the peripheral organs as a key mechanism to adjust the internal state of the body via the CNS. As an example, Qiao et al. recently showed how the central nervous system sensed bone density via prostaglandin E2 (PGE2) to regulate bone formation. Recent indicators have also shown the essential role of early inflammatory responses in Mg2+-induced new bone formation to stimulate macrophages (highly specialized immune cells that remove cellular debris) and create pro-osteogenic immune microenvironments.
Divalent cation-induced bone formation in mouse models
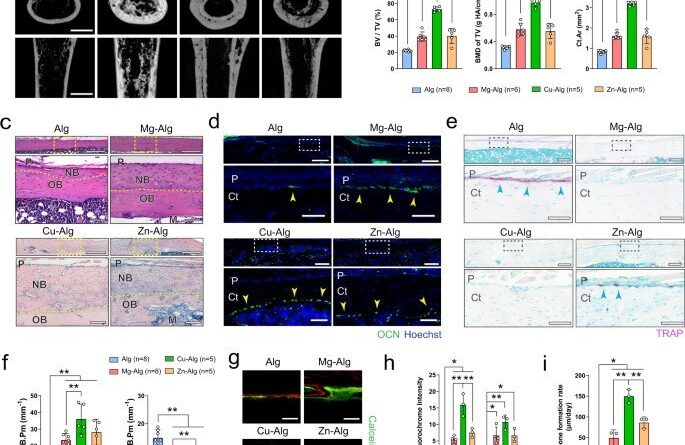
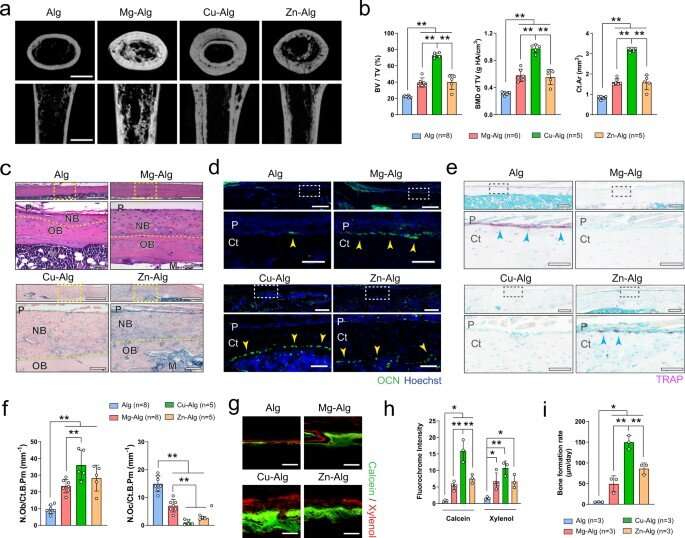
The team showed how divalent cations (Mg2+, Zn2+ and Cu2+) activated skeletal interoception via the immune-neural axis to regulate bone formation via the central nervous system. During early stage bone healing, the divalent cations induced PGE2 (prostaglandin E2) secretion from macrophages for bone formation via skeletal interoception. To observe this during experiments, Qiao et al. used an alginate-based hydrogel for localized delivery of the cations within mouse femurs, to investigate the effect of divalent cations on bone repair. Using micro-computed analysis, they showed substantial increase of bone volume fraction and bone mineral density in the femur four weeks after placing the divalent cation releasing alginate. They noted how the cortical bone area, cortical bone thickness and bone perimeter were greater in divalent cation-treated groups, compared with controls treated with pure alginate only. The trabecular thickness also increased in similarly treated femurs. Using hematoxylin and eosin dyes, the team showed significant bone formation at the peripheral cortex in divalent-cation treated femurs. Using immunofluorescence, they revealed an increased number of osteocalcin osteoblasts on femur surfaces treated with divalent cations. They then used fluorochrome labeling to show how divalent cations contributed to a significantly higher rate of mineral deposition and confirmed the non-toxicity of the divalent cations by histologically testing other organs including the liver, spleen, kidney and heart tissues during bone healing.
The mechanism of prostaglandin E2 (PGE2) production from macrophages
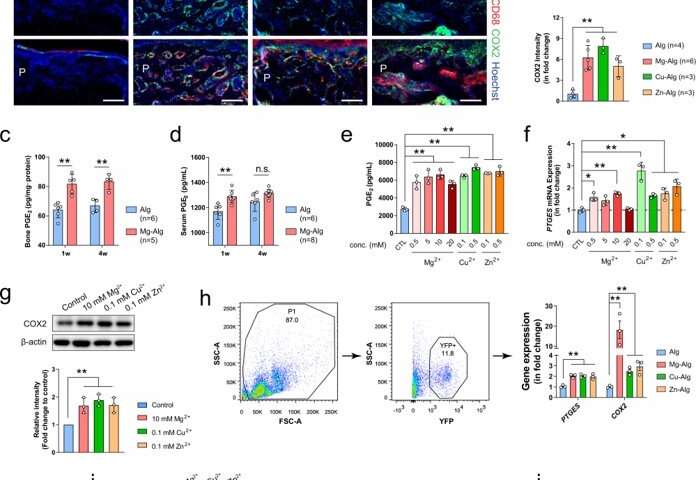
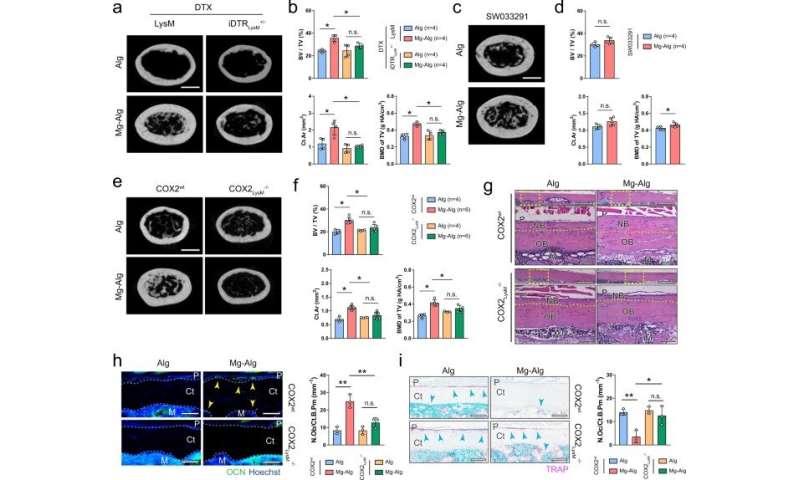
Qiao et al. further examined divalent cation-induced bone formation and characterized the immune response surrounding the cation-releasing alginate hydrogel. The experimental method promoted new bone formation during early inflammatory stages of bone healing, approximately seven days after the injury. The team showed how divalent cations stimulated the secretion of PGE2 from macrophages during inflammation, preceding bone healing. They then sought to understand the in vivo mechanism by developing a macrophage-depleted mouse model and accomplished this by injecting animals with the diphtheria toxin, which diminished the effect of Mg2+ on bone healing. When Qiao et al. next elevated the bone PGE2 level in the animal models, they noted that new bone formation in the femur improved significantly in the control and magnesium-alginate groups. They then developed mice with conditional knockout of the co-immunostaining cyclooxygenase (COX2) enzyme in macrophages to note how the cation-releasing hydrogel did not induce thickening of cortical bone in injured femurs of the experimental animal model, compared to the wild-type, to highlight the significance of COX2 on the immune-neural axis.
Sensory nerves are significant for divalent cation-induced bone formation
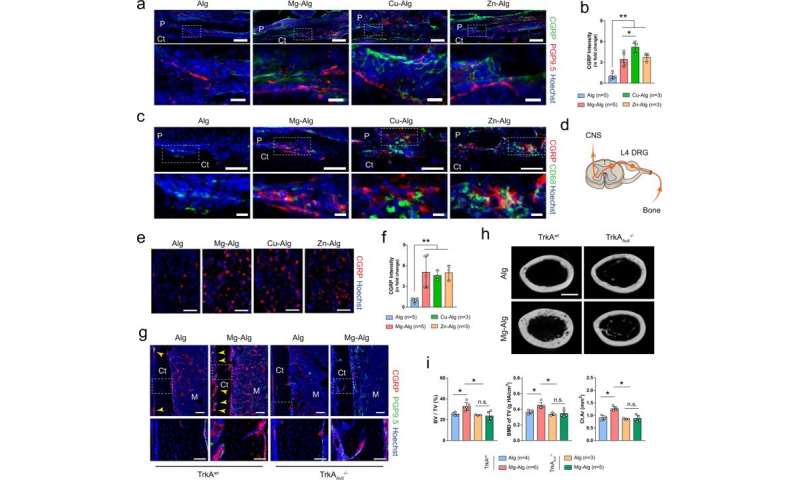
Qiao et al. further investigated the role of sensory nerves with divalent cation-induced bone formation by using immunostaining to identify the function of sensory nerve fibers. While they noted efficient sensory neuron interactions in wild-type native mice, such outcomes were not seen on the knockout mouse model. The team next created a sensory denervation mouse model to confirm the essential role of sensory nerves during divalent cation-induced bone formation. The results highlighted the importance of divalent cation-induced sensory innervation during new bone repair of injured bone tissue. The scientists similarly showed how divalent cations also promoted prostaglandin E2 (PGE2) from macrophages to activate skeletal interoception, further highlighting the neural mechanisms of new bone formation.
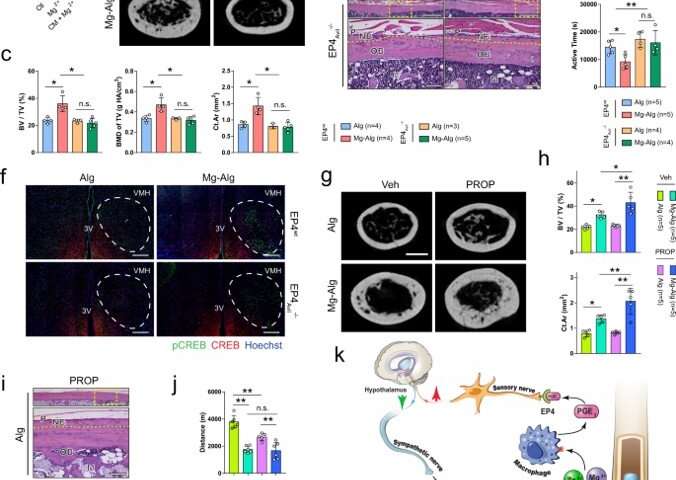
Outlook
Source: Read Full Article