A secret of developing life: In some instances the fetus helps repair a ruptured amniotic sac
Premature rupture of the amniotic sac can have devastating consequences, but scientists in Japan are studying instances when the damaged sac repairs itself—a phenomenon that requires cooperation of the developing fetus.
A new investigation of the amnion, the innermost layer of epithelial cells in the amniotic sac reveals a cascade of cellular events that result in sac repair. The new research helps lay the groundwork for a keener understanding of both healthy pregnancies and when things go awry.
Aware that premature rupture of the amniotic membranes can lead to premature birth, researchers at Kyoto University in Japan are asking what causes the sac to reseal. Producing a definitive answer to that question may ultimately lead to interventions that prevent premature birth for those whose membranes don’t reseal. The biggest potential benefit down the road is guarding against the sometimes lethal outcomes when babies are born too small, too soon.
“The premature rupture of the amniotic sac, a condition referred to as a preterm prelabor rupture of membranes (pPROM) is a leading cause of preterm birth. In some cases, these ruptured membranes heal spontaneously. We investigated repair mechanisms of the amnion, a layer of epithelial cells in the amniotic sac closest to the fetus,” writes Yosuke Kawamura in the journal Science Signaling.
Premature rupture of membranes affects an estimated 30% to 40% of preterm births, affecting about 150,000 women in the U.S. annually, according to Children’s Hospital of Philadelphia, where prematurity is also being studied. pPROM complicates 2% to 4% of all singleton births and 7% to 20% of twin pregnancies. Data show that women have experienced preterm, prelabor rupture of membranes—pPROM—in all trimesters of pregnancy.
Amniotic membranes normally remain intact until the onset of active labor or within 24 hours before labor starts. pPROM may not be easy to detect. For some women, fluid leaks slowly and may be mistaken for urine. Obstetricians say it’s important to note that amniotic fluid usually has no color and doesn’t smell like urine. They also underscore that premature membrane rupture can occur for a variety of reasons.
In some women the amnion can rupture early for reasons ranging from infections of the uterus, cervix, or vagina to too much stretching of the amniotic sac. The latter may occur as a direct result of too much fluid in the sac, or more than one baby putting pressure on the membranes. Other causes of membrane rupture include conditions such as malnutrition, or intrauterine bleeding. Smoking is another factor that can cause rupture.
Kawamura and colleagues turned to an animal model to better understand the phenomenon of resealing and how this protective event occurs. Their results answer at least one longstanding question in obstetric research and may help explain why when a rupture is suspected some women don’t experience preterm birth even because the rupture has somehow self-corrected.
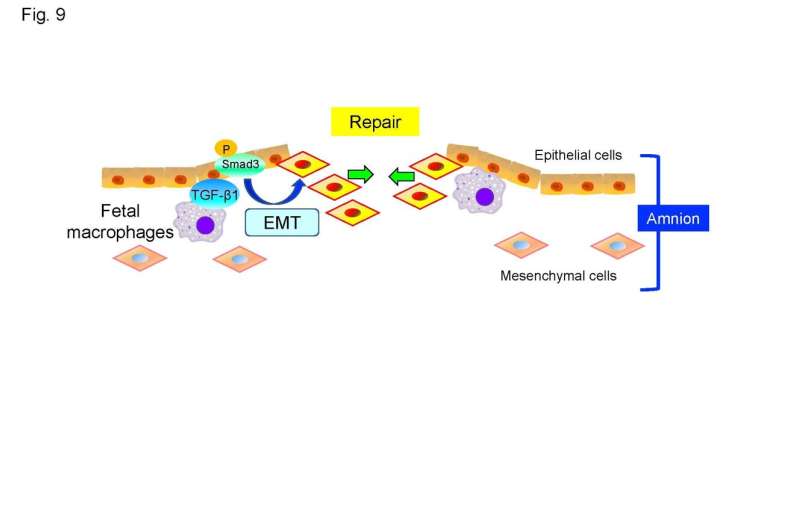
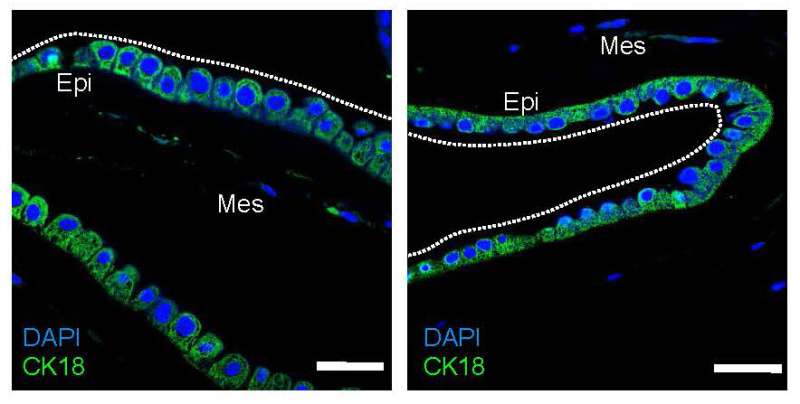
In a stunning series of findings, Kawamura and the Kyoto University team found that fetal macrophages are recruited to rupture sites in both the human and mouse amnion and assist in the repair of torn membranes.
“Macrophages migrated to and resided at rupture sites in both human and mouse amnion,” Kawamura added. “A process called epithelial-mesenchymal transition, in which epithelial cells acquire a mesenchymal phenotype and which is implicated in tissue repair, was observed at rupture sites.”
In short, epithelial cells took on the role of mesenchymal cells and helped begin the process of resealing. None of the cascade of biological events would have occurred without macrophages first migrating to and taking up residence at the rupture site.
Scientists previously thought ruptures were irreversible, but some women with preterm ruptures don’t go into labor and apparently show spontaneous repair of the ruptured membranes, factors that underlie the Kyoto research. Examining mice and ruptured human fetal membranes from six pregnancies, Kawamura and collaborators also observed amnion epithelial cells in laboratory wound-healing studies.
Amnion epithelial cells surrounding a rupture underwent epithelial-mesenchymal transition, a process that enhances cell migration and tissue repair. The team also found that depleting macrophages in mouse fetuses compromised amnion repair, suggesting that fetal macrophages are needed to repair the amnion after a preterm rupture.
Their research also revealed that membrane repair is highly reliant on signaling molecules: in this case, transformation growth factor-β/Smad—TGF-β/Smad—signaling. TGF-β/Smad was prominent in both mouse and human samples.
“We conclude that amnion has high regenerative potential through EMT of amnion epithelial cells and that fetal macrophages are important in mediating this wound-repairing process,” Kawamura and the Kyoto team asserted.
They speculate that their study’s findings could potentially inform research into treatments for preterm membrane ruptures to prevent preterm birth.
More information:
Yosuke Kawamura et al, Fetal macrophages assist in the repair of ruptured amnion through the induction of epithelial-mesenchymal transition, Science Signaling (2022). DOI: 10.1126/scisignal.abi5453
Journal information:
Science Signaling
Source: Read Full Article