A ‘backpack’ full of multiple sclerosis therapy
Multiple sclerosis (MS) is a devastating autoimmune disease that destroys the protective myelin covering around nerves, disrupting communication between the brain and body, and causing patients’ ability to move and function to progressively decline.
The MS atlas reported in 2020 that someone is diagnosed with MS every five minutes around the world, adding to about 2.8 million individuals that currently have to live with the disease. Alarmingly, since 2013, the world-wide prevalence of MS has risen by 30%.
A key driver of MS is the sudden inflammation of nerves caused by so-called myeloid cells of the “innate” immune system in vulnerable regions of the brain and spinal cord, which together form the central nervous system (CNS). These “acute inflammatory lesions” then attract other myeloid cells, as well as self-reactive T and B cells that belong to the immune system’s second arm, known as the “adaptive immune system” and directly attack the myelin covering.
While no cure is available for MS, existing disease-modifying therapies in the form of small molecule and protein drugs either directly target the self-reactive immune cells or broadly dampen inflammation. However, many of those therapies cause severe side effects in different parts of the body, including the immune system itself, and thus carry significant health risks.
Now, a research team at the Wyss Institute for Biologically Inspired Engineering at Harvard University and Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) has developed a cell therapy as a strong alternative to existing small molecule and protein therapies that leverages myeloid cells, the very type of immune cells that cause the MS-triggering nerve inflammation in patients.
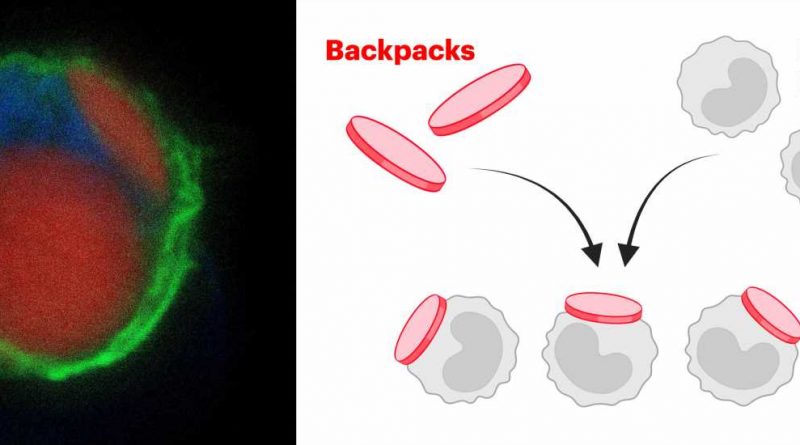
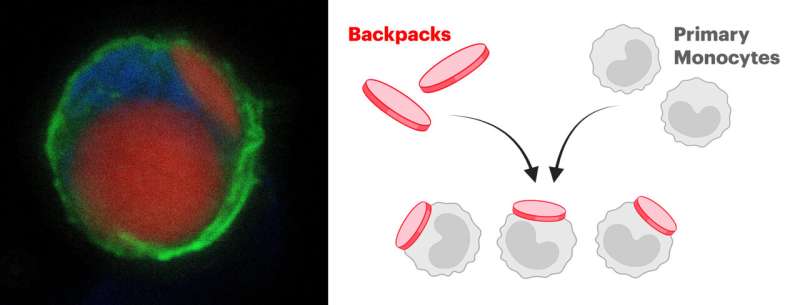
To transform potentially inflammatory myeloid cells into therapeutic cells, they isolated and cultured monocytes (a type of myeloid cell) from the bone marrow of donor mice and stably attached tiny microparticles, termed “backpacks,” to the cells’ surfaces. These backpacks are loaded with anti-inflammatory molecules that direct the carrier cells’ differentiation into anti-inflammatory cells in vivo.
When infused back into a mouse model of MS, the backpack-laden monocytes were able to affect MS-specific immune responses, and partially reverse hind limb paralysis and improve motor functions. The results are published in the Proceedings of the National Academy of Sciences (PNAS).
“Current MS therapies do not specifically target myeloid cells. These are very plastic cells that can toggle between different states and are thus hard to control. Our biomaterial-based backpack approach is a highly effective way to keep them locked into their anti-inflammatory state,” said senior author Samir Mitragotri, Ph.D., who is a Core Faculty member at the Wyss Institute.
“In many ways simpler than other cell therapies, myeloid cells can be easily obtained from patients’ peripheral blood, modified with backpacks in a short culture step, and reinfused back into the original donor, where they find their way to inflammatory lesions and affect the MS-specific immune response not only locally, but more broadly.” Mitragotri is also the Hiller Professor of Bioengineering and Hansjörg Wyss Professor of Biologically Inspired Engineering at SEAS.
Many cell therapies, such as the famed CAR-T cell therapies, require the mobilization of immune cells from specific tissue compartments in the body with drugs, genetic modification, and then amplification over weeks outside of the body. Myeloid cells can be directly retrieved using established methods and modified with backpacks within hours, making the therapy more easily translatable. In addition, some myeloid cell types possess the ability to traverse the blood-brain barrier, which makes them particularly suitable for treating CNS diseases.
New spin for cellular backpacks
Mitragotri’s group had previously found that when they attached small disc-shaped backpacks to cells of the myeloid lineage, they remained stably exposed on the cells’ surface, whereas many other cells would readily internalize and inactivate them. Adding certain molecules to the backpacks allowed the team sustained control over the cells’ behavior.
They made use of this finding in a tumor-fighting cell therapy consisting of backpack-laden macrophages, which is a specific type of myeloid cell. In their new study, they focused on monocytes, which also belong to the myeloid differentiation lineage and are a precursor to macrophages. Monocytes can effectively infiltrate the brain and then differentiate into macrophages, which are one of the predominant inflammatory cell types in active MS lesions.
“Because of their ability to invade the CNS, infiltrate inflammatory lesions, and differentiate into macrophages, a backpack strategy allowing control over monocyte differentiation made extreme sense,” said first author Neha Kapate, a graduate student working with Mitragotri. “We decided on backpacks that contained interleukin-4 [IL-4] and dexamethasone, two molecules that we later found to provide a synergistic anti-inflammatory effect.”
The team fabricated their micrometer-size backpacks via a process known as serial “spin coating,” in which thin films made up of a PLGA polymer and other biocompatible substances, and containing the anti-inflammatory molecules are layered on top of each other like layers of an onion. As a final step, the outer surface of the backpack was furnished with an antibody fragment to allow it to stick to monocytes.
Cellular backpacks get legs
To test the backpack-laden monocytes for their therapeutic efficacy, the researchers isolated monocytes from healthy donor mice and, in a short cell culture step, attached the backpacks to them. They then infused the modified cells into a mouse model of MS, known among researchers as experimental autoimmune encephalomyelitis (EAE) model.
“When we infused backpack-carrying monocytes and, in parallel, unaltered control monocytes into EAE mice with ongoing nerve inflammation, backpack-carrying monocytes more effectively infiltrated into inflamed CNS lesions. They also reduced inflammation inside the lesions and shifted the local and systemic MS-associated immune response towards a therapeutic outcome,” said Kapate.
“The resulting anti-inflammatory monocytes also elicited cross-talk effects with other immune cell populations, such as specific T helper cells that are linked to the self-directed adaptive auto-immune response.”
The disease symptoms in EAE mice treated with backpack-laden monocytes were significantly improved and, by the end of the study, the animals merely exhibited a limp tail, compared to complete a paralysis in the control animals’ hind limbs. The treatment also extended the animals’ survival—all mice receiving backpack-carrying monocytes survived to the end of the study, whereas a significant number of the control mice had died.
Importantly, the magnitude of therapeutic benefit the team observed is on par with reported therapeutic treatments that had been tested in other studies using the same model.
Since the EAE model mainly mimics the progressive form of MS and not the more prevalent “relapsing-remitting” form, with which the disease begins in about 85% of MS patients, and which at later stages can also become progressive, the team plans to also investigate their approach in models of relapsing-remitting MS. Being able to suppress inflammation early on could have enormous benefits for patients.
“The ability of this team to convert a potentially pathogenic type of immune cell into a therapeutic one for MS, which is extremely hard or impossible to treat, could open an entirely new path to treat patients with a variety of neurological diseases,” said Wyss Founding Director Donald Ingber.
More information:
Neha Kapate et al, A backpack-based myeloid cell therapy for multiple sclerosis, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2221535120
Journal information:
Proceedings of the National Academy of Sciences
Source: Read Full Article