Cooperation between muscle and liver circadian clocks is key to controlling glucose metabolism, finds study
Collaborative work by teams at the Department of Medicine and Life Sciences (MELIS) at Pompeu Fabra University (UPF), University of California, Irvine (UCI), and the Institute for Research in Biomedicine (IRB Barcelona) has shown that interplay between circadian clocks in liver and skeletal muscle controls glucose metabolism.
The findings reveal that local clock function in each tissue is not enough for whole-body glucose metabolism but also requires signals from feeding and fasting cycles to properly maintain glucose levels in the body. Understanding the components underlying glucose balance has clear implications for metabolic diseases such as diabetes or other age-related disorders.
Circadian clocks are present in virtually every cell in the body. They align biological processes to a 24-hour cycle to synchronize physical, mental, and behavioral changes. This process is supported by the central clock in the brain that synchronizes clocks in peripheral tissues. “Maintenance of circadian rhythms is related to general health when robust, but to disease when disrupted. So, circadian disturbances can affect carbohydrate metabolism and induce diabetes-like abnormalities,” explains Pura Muñoz-Cánoves, senior author of the study at MELIS-UPF.
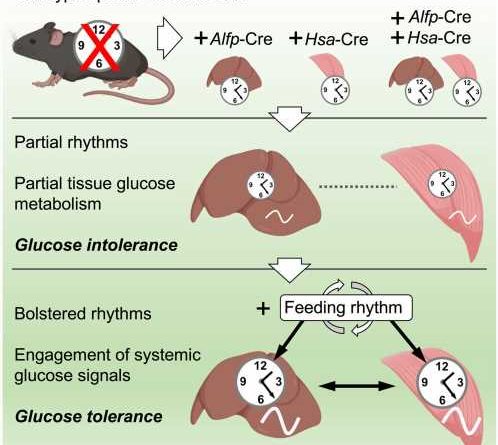
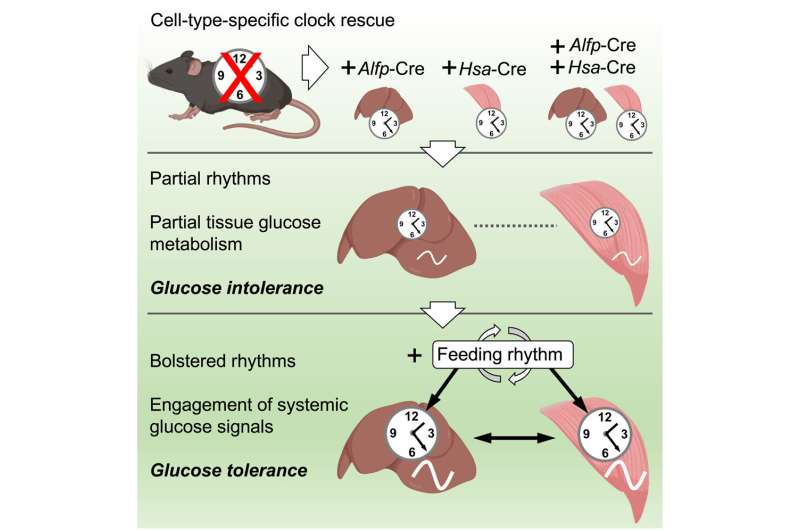
The study published June 1 in Cell Reports demonstrates, surprisingly, that clocks in liver and muscle can keep time on their own in the absence of the central clock in the brain, although the strength of their rhythms is reduced.
The study also found that in these conditions, glucose uptake and processing levels were altered. However, combining the clocks with feeding-fasting cycles improved the function of each of the clocks and restored glucose regulation in the combined system. This finding demonstrates that a daily feeding-fasting rhythm is key to the synergy of the liver and muscle clocks and to the restoration of glucose metabolic control.
Jacob Smith, a postdoctoral researcher at MELIS-UPF who co-led the study with Kevin Koronowski, commented, “Our study reveals that a minimal clock network is needed for glucose tolerance. The central clock, which controls daily feeding cycles, cooperates with local clocks in liver and muscle. Now, the next step is to identify the signaling factors involved in this interaction.”
“We believe this finding may hold promise for the treatment of human diseases such as diabetes, in which this liver-muscle network may be targeted for therapeutic gain, and for other age-related disorders,” adds Muñoz-Cánoves, who is now also a principal investigator at Altos Labs in San Diego.
The findings have been achieved using a “clockless” mouse model, developed in the laboratory of Salvador AznarBenitah at IRB Barcelona, in which they have restored clocks only in liver or skeletal muscle or combined clocks in both organs.
“This is a great example of how by studying communication between peripheral tissues one starts to understand the complex interplay of how systemic communication takes place. We are so thrilled to see how the daily coordination between the liver and the muscle was capable of sustaining systemic glucose tolerance, something that was completely unexpected by us,” explains Salvador Aznar Benitah, ICREA researcher and head of the Stem Cells and Cancer lab at IRB Barcelona.
This collaborative study was initiated in the laboratory of the late Paolo Sassone-Corsi at UCI and has been supported by the work of the laboratories of Selma Masri, Cholsoon Jang and Pierre Baldi at UCI. As Salvador Aznar-Benitah, commented, “this work is a testament to the collaborative and ground-breaking science that Paolo was known for.”
More information:
Jacob G. Smith et al, Liver and muscle circadian clocks cooperate to support glucose tolerance in mice, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.112588
Journal information:
Cell Reports
Source: Read Full Article