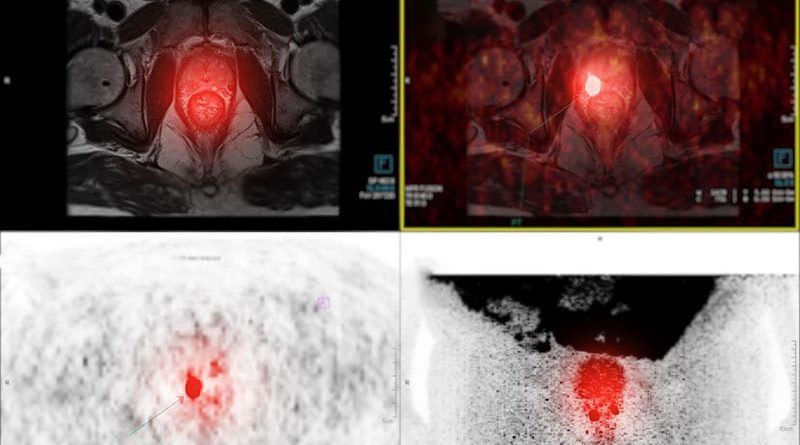
Posluma Approved for PET Imaging in Prostate Cancer
The US Food and Drug Administration (FDA) has approved flotufolastat fluorine-18 (Posluma), a radiopharmaceutical for use with positron-emission tomography (PET) in prostate cancer.
The product is approved for use in men with suspected metastasis who are candidates for definitive therapy and for men with suspected recurrence, as evidenced by elevations in serum prostate-specific antigen (PSA) level, according to a May 30 press release from marketer Blue Earth Diagnostics.
Posluma binds prostate-specific membrane antigen (PSMA), which is usually overexpressed on prostate cancer cells, and tags the cells with fluorine-18 (F18), a positron emitter. Because of the radiolabeling, PET imaging can be used to gauge the extent of disease.
Posluma will be available in the United States in June 2023 from Blue Earth’s US manufacturer and distributor, PETNET Solutions.
Blue Earth says that its new agent, which was known as 18F-rhPSMA-7.3 PET during trials, “is the first and only FDA-approved, PSMA-targeted imaging agent developed with proprietary radiohybrid technology.”
However, a similar product is currently on the US market ― the PSMA PET imaging radiopharmaceutical gallium-68 gozetotide (Illuccix, Locometz), which has the same two indications. Gozetotide is also indicated for metastatic prostate cancer amenable to lutetium Lu 177 vipivotide tetraxetan PSMA-directed therapy.
Approval Based on Two Single-Arm Trials
Posluma’s approval was based on two single-arm trials from Blue Earth.
In the LIGHTHOUSE trial, 296 men underwent Posluma PET imaging before radical prostatectomy with pelvic lymph node dissection. About a quarter turned out to have positive nodes on pathology.
Posluma’s sensitivity for predicting positive nodes was low, ranging from 23% to 30% among three readers who were blinded to clinical information, but its specificity was high, ranging from 93% to 97%, according to the product labeling.
“The study showed that Posluma PET provided clinically valuable information prior to surgery that would likely result in management changes for these patients,” said investigator Brian Chapin, MD, a urologist at MD Anderson Cancer Center, Houston, in the company press release.
The second trial, SPOTLIGHT, included 389 men suspected of experiencing recurrence on the basis of elevations in PSA.
Posluma PET’s ability to detect true recurrence was compared with use of histology or other imaging techniques, including CT, MRI, technetium 99m bone scan, and fluciclovine F18 PET. In regions deemed positive for recurrence on Posluma PET by three readers, 46% to 60% were positive by the other techniques, the labeling says.
Overall, the “results demonstrated high detection rates…even at low PSA levels,” Blue Earth said.
Adverse events were minimal in the trials. The most frequent were diarrhea (0.7%), increases in blood pressure (0.5%), and injection site pain (0.4%).
The product labeling warns that Posluma PET contributes to patients’ overall long-term cumulative radiation exposure and that interpretation with respect to recurrence may differ among readers.
The labeling also cautions that “a negative image does not rule out the presence of prostate cancer and a positive image does not confirm the presence of prostate cancer…. Uptake is not specific for prostate cancer and may occur in other types of cancer, in non-malignant processes, and in normal tissues.”
In addition, it notes that androgen deprivation therapy “and other therapies targeting the androgen pathway, such as androgen receptor antagonists, may result in changes in uptake of flotufolastat F 18 in prostate cancer.”
The labeling for gozetotide carries the same warnings and precautions.
M. Alexander Otto is a physician assistant with a master’s degree in medical science and a journalism degree from Newhouse. He is an award-winning medical journalist who worked for several major news outlets before joining Medscape. Alex is also an MIT Knight Science Journalism fellow. Email: [email protected]
Follow Medscape on Facebook, Twitter, Instagram, and YouTube. Have a tip for us? Contact us.
Source: Read Full Article