Active ingredients in sunscreen may be absorbed into the bloodstream, FDA says
The active ingredients in sunscreen may soak all the way into the user’s bloodstream, according to the results of a study released by the Food and Drug Administration.
The study, published in the Journal of the American Medical Association on Monday, evaluated the systemic absorption of four commercially available sunscreens. Researchers found that at least one active ingredient was absorbed in significant amounts in all four formulas tested, according to the FDA.
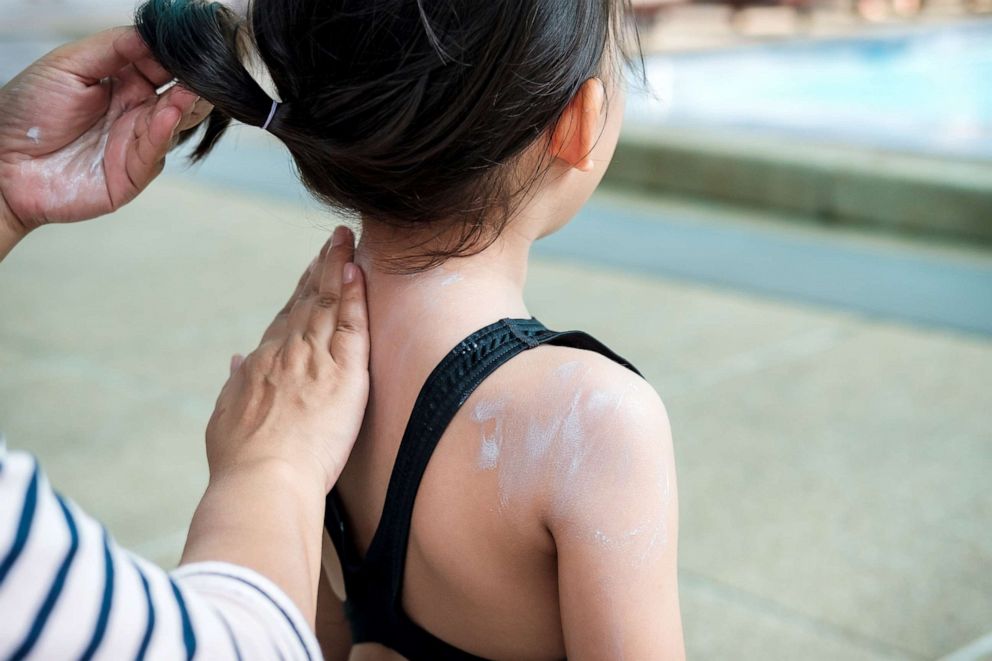
The fact that an ingredient is absorbed through the skin and into the body does not mean the ingredient is unsafe, and the public should continue to use sunscreens of at least 15 SPF along with other protective measures, such as sunglasses and hats, according to the FDA.
“Skin cancer incidence rates continue to rise, making risk from excess sun exposure an important public health priority,” according to the agency.

The study shows that further testing is needed to determine the safety of the absorbed ingredients for repeated use.
Last month, the FDA announced that mineral sunscreen ingredients, specifically zinc oxide and titanium dioxide, are safe, but another 12 ingredients commonly used in sunscreens, including oxybenzone and octinoxate, need more research to determine if they can be officially listed as safe.
The FDA is asking the industry for additional safety data on those 12 ingredients. The agency began regulating sunscreens in the 1970s.
ABC News’ Stephanie Ebbs and Eric Strauss contributed to this report.
Source: Read Full Article